Double-blinded infliximab dose escalation in patients with rheumatoid arthritis
- PMID: 17392352
- PMCID: PMC2652128
- DOI: 10.1136/ard.2006.065995
Double-blinded infliximab dose escalation in patients with rheumatoid arthritis
Abstract
Objective: To determine the efficacy, safety and pharmacokinetics of infliximab dose escalation in patients with rheumatoid arthritis (RA) who had an inadequate response to 3 mg/kg infliximab treatment or whose disease flared after initially responding.
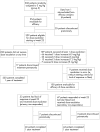
Methods: Patients with active RA, despite receiving methotrexate, received infliximab 3 mg/kg at weeks 0, 2, 6 and 14 in one of the three arms of the START trial. Beginning at week 22, patients had their infliximab dose increased in a double-blind fashion in increments of 1.5 mg/kg if the total tender and swollen joint count did not improve by at least 20% from baseline (lack of response) or the improvement at week 22 or later worsened by 50% or more (criterion for flare).
Results: Of the 329 evaluable patients, 100 (30.4%) patients required dose escalation at or after week 22 because of flare or lack of response. The majority of patients (>80%) who received up to three dose escalations showed >/=20% improvement in the total tender and swollen joint count after their last dose escalation. Patients who required dose escalations generally had lower preinfusion serum infliximab concentrations than those who did not require them. The incidences of adverse events and serious adverse events for the patients who received dose escalation(s) were similar to those of patients who did not receive dose escalation.
Conclusion: Fewer than one-third of patients required a dose escalation. The majority of patients showed improvement after receiving increased doses of infliximab, without an increased risk of adverse events.
Conflict of interest statement
Drs Rahman, Baker, Wagner and Han are employees of Centocor Research and Development, Inc, a wholly owned subsidiary of Johnson and Johnson, Inc, and hold Johnson and Johnson stock. Dr Westhovens has received consulting fees from Schering‐Plough Belgium and Bristol‐Myers Squibb and has received speaking fees from Abbott and UCB. Dr Yocum has received grants and speaking fees from Centocor, Amgen and Abbott and has received consulting fees from Centocor. Dr Yocum is currently an employee of Genentech. Drs Strusberg, Geusens and Berman have no potential conflicts to declare.
References
-
- Maini R, St Clair E W, Breedveld F, Furst D, Kalden J, Weisman M.et al Infliximab (chimeric anti‐tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 19993541932–1939. - PubMed
-
- Maini R N, Breedveld F C, Kalden J R, Smolen J S, Furst D, Weisman M H.et al Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum 2004501051–1065. - PubMed
-
- Remicade [package insert] Malvern, PA: Centocor, Inc
-
- Durez P, Van den Bosch F, Corluy L, Veys E M, De Clerck L, Peretz A.et al A dose adjustment in patients with rheumatoid arthritis not optimally responding to a standard dose of infliximab of 3 mg/kg every 8 weeks can be effective: a Belgian prospective study. Rheumatology (Oxford) 200544465–468. - PubMed