The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation
- PMID: 17392452
- PMCID: PMC6672109
- DOI: 10.1523/JNEUROSCI.4833-06.2007
The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation
Abstract
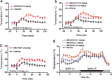
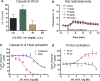
The vanilloid receptor TRPV1 (transient receptor potential vanilloid 1) is a cation channel that serves as a polymodal detector of pain-producing stimuli such as capsaicin, protons (pH <5.7), and heat. TRPV1 antagonists block pain behaviors in rodent models of inflammatory, neuropathic, and cancer pain, suggesting their utility as analgesics. Here, we report that TRPV1 antagonists representing various chemotypes cause an increase in body temperature (hyperthermia), identifying a potential issue for their clinical development. Peripheral restriction of antagonists did not eliminate hyperthermia, suggesting that the site of action is predominantly outside of the blood-brain barrier. Antagonists that are ineffective against proton activation also caused hyperthermia, indicating that blocking capsaicin and heat activation of TRPV1 is sufficient to produce hyperthermia. All TRPV1 antagonists evaluated here caused hyperthermia, suggesting that TRPV1 is tonically activated in vivo and that TRPV1 antagonism and hyperthermia are not separable. TRPV1 antagonists caused hyperthermia in multiple species (rats, dogs, and monkeys), demonstrating that TRPV1 function in thermoregulation is conserved from rodents to primates. Together, these results indicate that tonic TRPV1 activation regulates body temperature.
Figures


References
-
- Acs G, Palkovits M, Blumberg PM. Specific binding of [3H]resiniferatoxin by human and rat preoptic area, locus ceruleus, medial hypothalamus, reticular formation and ventral thalamus membrane preparations. Life Sci. 1996;59:1899–1908. - PubMed
-
- Bannon AW, Davis JR, Zhu D, Norman MH, Doherty E, Magal E, Treanor J. Involvement of trpv1 in the regulation of body temperature in rats and mice. Soc Neurosci Abstr. 2004;30:890.24.
-
- Boulant JA. Hypothalamic mechanisms in thermoregulation. Fed Proc. 1981;40:2843–2850. - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
-
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
