Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain
- PMID: 17392456
- PMCID: PMC6672128
- DOI: 10.1523/JNEUROSCI.4605-06.2007
Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain
Abstract
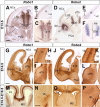
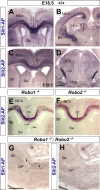
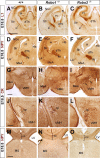
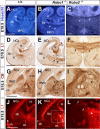
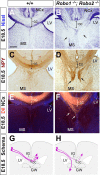
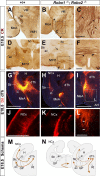
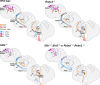
The function of the nervous system depends on the precision of axon wiring during development. Previous studies have demonstrated that Slits, a family of secreted chemorepellent proteins, are crucial for the proper development of several major forebrain tracts. Mice deficient in Slit2 or, even more so, in both Slit1 and Slit2 have defects in multiple axonal pathways, including corticofugal, thalamocortical, and callosal connections. In the spinal cord, members of the Robo family of proteins help mediate the function of Slits, but the relative contribution of these receptors to the guidance of forebrain projections remains to be determined. In the present study, we addressed the function of Robo1 and Robo2 in the guidance of forebrain projections by analyzing Robo1-, Robo2-, and Robo1;Robo2-deficient mice. Mice deficient in Robo2 and, more dramatically, in both Robo1 and Robo2, display prominent axon guidance errors in the development of corticofugal, thalamocortical, and corticocortical callosal connections. Our results demonstrate that Robo1 and Robo2 mostly cooperate to mediate the function of Slit proteins in guiding the major forebrain projections.
Figures




References
-
- Andrews W, Liapi A, Plachez C, Camurri L, Zhang J, Mori S, Murakami F, Parnavelas JG, Sundaresan V, Richards LJ. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–2252. - PubMed
-
- Auladell C, Pérez-Sust P, Supèr H, Soriano E. The early development of thalamocortical and corticothalamic projections in the mouse. Anat Embryol. 2000;201:169–179. - PubMed
-
- Bagri A, Marín O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. - PubMed
-
- Braisted JE, Tuttle R, O'Leary DD. Thalamocortical axons are influenced by chemorepellent and chemoattractant activities localized to decision points along their path. Dev Biol. 1999;208:430–440. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
