Myelination of congenitally dysmyelinated spinal cord axons by adult neural precursor cells results in formation of nodes of Ranvier and improved axonal conduction
- PMID: 17392458
- PMCID: PMC6672112
- DOI: 10.1523/JNEUROSCI.0273-07.2007
Myelination of congenitally dysmyelinated spinal cord axons by adult neural precursor cells results in formation of nodes of Ranvier and improved axonal conduction
Abstract
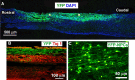
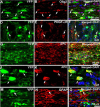
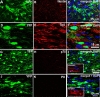
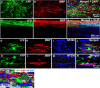
Emerging evidence suggests that cell-based remyelination strategies may be a feasible therapeutic approach for CNS diseases characterized by myelin deficiency as a result of trauma, congenital anomalies, or diseases. Although experimental demyelination models targeted at the transient elimination of oligodendrocytes have suggested that transplantation-based remyelination can partially restore axonal molecular structure and function, it is not clear whether such therapeutic approaches can be used to achieve functional remyelination in models associated with long-term, irreversible myelin deficiency. In this study, we transplanted adult neural precursor cells (aNPCs) from the brain of adult transgenic mice into the spinal cords of adult Shiverer (shi/shi) mice, which lack compact CNS myelin. Six weeks after transplantation, the transplanted aNPCs expressed oligodendrocyte markers, including MBP, migrated extensively along the white matter tracts of the spinal cord, and formed compact myelin. Conventional and three-dimensional confocal and electron microscopy revealed axonal ensheathment, establishment of paranodal junctional complexes leading to de novo formation of nodes of Ranvier, and partial reconstruction of the juxtaparanodal and paranodal molecular regions of axons based on Kv1.2 and Caspr (contactin-associated protein) expression by the transplanted aNPCs. Electrophysiological recordings revealed improved axonal conduction along the transplanted segments of spinal cords. We conclude that myelination of congenitally dysmyelinated adult CNS axons by grafted aNPCs results in the formation of compact myelin, reconstruction of nodes of Ranvier, and enhanced axonal conduction. These data suggest the therapeutic potential of aNPCs to promote functionally significant myelination in CNS disorders characterized by longstanding myelin deficiency.
Figures




Comment in
-
Adult neural precursor cells and the dysmyelinated spinal cord.J Neurosci. 2007 Jun 20;27(25):6605-6. doi: 10.1523/JNEUROSCI.2125-07.2007. J Neurosci. 2007. PMID: 17581947 Free PMC article. Review. No abstract available.
References
-
- Baba H, Akita H, Ishibashi T, Inoue Y, Nakahira K, Ikenaka K. Completion of myelin compaction, but not the attachment of oligodendroglial processes triggers K+-channel clustering. J Neurosci Res. 1999;58:752–764. - PubMed
-
- Baron-Van Evercooren A, Clerin-Duhamel E, Lapie P, Gansmuller A, Lachapelle F, Gumpel M. The fate of Schwann cells transplanted in the brain during development. Dev Neurosci. 1992;14:73–84. - PubMed
-
- Bird TD, Farrell DF, Sumi SM. Brain lipid composition of the shiverer mouse (genetic defect in myelin development) J Neurochem. 1978;31:387–391. - PubMed
-
- Black JA, Waxman SG, Smith KJ. Remyelination of dorsal column axons by endogenous Schwann cells restores the normal pattern of Nav1.6 and Kv1.2 at nodes of Ranvier. Brain. 2006;129:1319–1329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
