Ataxin-2 interacts with the DEAD/H-box RNA helicase DDX6 and interferes with P-bodies and stress granules
- PMID: 17392519
- PMCID: PMC1838996
- DOI: 10.1091/mbc.e06-12-1120
Ataxin-2 interacts with the DEAD/H-box RNA helicase DDX6 and interferes with P-bodies and stress granules
Abstract
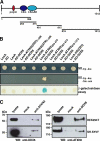
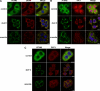
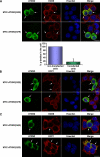
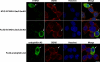
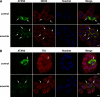
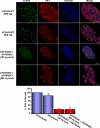
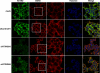
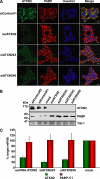
Tight control of translation is fundamental for eukaryotic cells, and deregulation of proteins implicated contributes to numerous human diseases. The neurodegenerative disorder spinocerebellar ataxia type 2 is caused by a trinucleotide expansion in the SCA2 gene encoding a lengthened polyglutamine stretch in the gene product ataxin-2, which seems to be implicated in cellular RNA-processing pathways and translational regulation. Here, we substantiate a function of ataxin-2 in such pathways by demonstrating that ataxin-2 interacts with the DEAD/H-box RNA helicase DDX6, a component of P-bodies and stress granules, representing cellular structures of mRNA triage. We discovered that altered ataxin-2 levels interfere with the assembly of stress granules and cellular P-body structures. Moreover, ataxin-2 regulates the intracellular concentration of its interaction partner, the poly(A)-binding protein, another stress granule component and a key factor for translational control. Thus, our data imply that the cellular ataxin-2 concentration is important for the assembly of stress granules and P-bodies, which are main compartments for regulating and controlling mRNA degradation, stability, and translation.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

