Control of PDGF-induced reactive oxygen species (ROS) generation and signal transduction in human lens epithelial cells
- PMID: 17392688
- PMCID: PMC2633485
Control of PDGF-induced reactive oxygen species (ROS) generation and signal transduction in human lens epithelial cells
Abstract
Purpose: The mitogenic action of PDGF has been shown to associate with reactive oxygen species (ROS) generation, but the mechanism leading to ROS production and subsequent cell proliferation is not clear. We investigated the upstream membrane-bound target proteins involved in PDGF-stimulated signal transduction in human lens epithelial cell (HLE B3), using specific inhibitors and transfected cells.
Methods: PDGF (1 ng/ml)-stimulated ROS generation was measured using fluorescent reaction of DCFDA by confocal microscope in live HLE B3 cells. Western blot analysis was used to determine the activated MAP kinases in cell lysates. Specific inhibitors used in this study were: AG1296 for PDGF receptor (PDGFR); AG1517 for EGF receptor (EGFR); pertussis toxin for cytokine-binding G protein coupled receptor (GPCR); PP1 for Src-family kinases; LY294002 for phosphatidylinositol-3 kinase (PI3K). Small GTP-binding proteins Rac and Ras were studied using transfectants of dominant negative Rac (Rac N17), Ras (Ras N17) or constitutively active Rac (Rac V12). Cell proliferation was quantified using BrdU incorporation method.
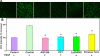
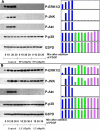
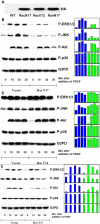
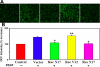
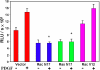
Results: Inhibitions of PDGF receptor kinase, the docking protein component Src-family kinases, and the survival element PI3K all eradicated PDGF-stimulated ROS production and corroborated with the suppressed cell growth. These inhibitions also attenuated the activated ERK1/2, JNK, and Akt, all downstream targets of the above factors. Interestingly, inhibiting GPCR or EGFR also showed the same effect but to a lesser degree. Co-inhibiting receptors to PDGF and EGF with or without co-inhibiting GPCR eradicated the PDGF signaling system completely. Transiently transfected cells with plasmid from small GTP-binding proteins Rac N17 or Ras N17 diminished PDGF action in ROS generation, cell proliferation and MAP kinase activation, while cells with Rac V12 enhanced the PDGF effect.
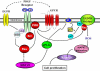
Conclusions: Our data clarified the potential mechanism of PDGF signaling in the lens epithelial cells, in which concerted efforts of the upstream components of PDGF receptor kinase, Src-family kinases, PI3K, Rac, and Ras proteins are required. This report also provided novel findings that GPCR and EGF receptors may control PDGF signaling in the lens epithelial cells via integrative signaling and transactivation mechanisms, respectively.
Figures


References
-
- Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227:375–81. - PubMed
-
- Chen Z, Silva H, Klessig DF. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science. 1993;262:1883–6. - PubMed
-
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–93. - PubMed
-
- Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18:775–94. - PubMed
-
- Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–53. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
