Revascularization and remodelling of pancreatic islets grafted under the kidney capsule
- PMID: 17394557
- PMCID: PMC2375740
- DOI: 10.1111/j.1469-7580.2007.00717.x
Revascularization and remodelling of pancreatic islets grafted under the kidney capsule
Abstract
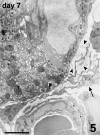
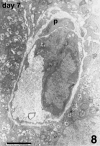
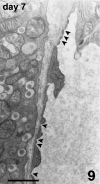
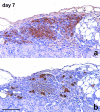
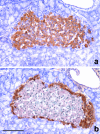
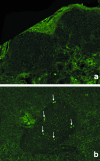
The revascularization and the structural changes resulting from interactions between the graft and the host were investigated in transplanted pancreatic islets under the kidney capsule. Islets were isolated from mice pancreata and transplanted in syngeneic diabetic animals. Graft-bearing kidneys were collected on different days post-transplant and processed for light microscopy, immunohistochemistry and transmission electron microscopy. A numerical analysis was performed in order to compare the percentage number of the different types of cells in native islets and at different time points after the transplant. Recipient animals reversed diabetes within 4 days. An intraperitoneal glucose tolerance test was performed to determine islet functionality under stressful conditions. During the initial few days post-transplant, the islets showed peculiar shapes and the graft tended to aggregate along the vessels. Starting at days 4-7 post-transplant, islets were revascularized from vessels connected to both the cortical and the capsular vascular network of the kidney. From day 7-14 post-transplant, the vessels progressively appeared more similar in features and size to those of in situ pancreatic islets. Both the percentage number of the different cell types and the distribution of Alpha, Beta and Delta cells inside the graft were significantly different as compared with intact islets, demonstrating quantitative and structural changes after the engraftment. No concomitant proliferation of Beta cells was detected using a bromodeoxyuridin staining method. Despite the fact that quick revascularization preserved a large mass of tissue, the remodelling process of the graft and the newly formed vascularization led to a different organization of the endocrine tissue as compared with intact in situ islets. This constitutes the morphological basis for alterations of the normal intercellular interactions and may explain the altered secretory cell function often observed in transplant.
Figures
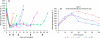






Similar articles
-
Morphological changes of isolated rat pancreatic islets: a structural, ultrastructural and morphometric study.J Anat. 2006 Sep;209(3):381-92. doi: 10.1111/j.1469-7580.2006.00620.x. J Anat. 2006. PMID: 16928206 Free PMC article.
-
Islet engraftment and revascularization in clinical and experimental transplantation.Cell Transplant. 2013;22(2):243-51. doi: 10.3727/096368912X640637. Epub 2012 May 8. Cell Transplant. 2013. PMID: 22584061
-
Peripheral mobilization of recipient bone marrow-derived endothelial progenitor cells enhances pancreatic islet revascularization and engraftment after intraportal transplantation.Surgery. 2003 Aug;134(2):390-8. doi: 10.1067/msy.2003.250. Surgery. 2003. PMID: 12947346
-
[Severe vascular dysfunction shown in transplanted islets].Lakartidningen. 2003 Apr 3;100(14):1223-8. Lakartidningen. 2003. PMID: 12756650 Review. Swedish.
-
[Experimental and clinical islets transplantation. Current status].Zentralbl Chir. 1992;117(12):670-6. Zentralbl Chir. 1992. PMID: 1285474 Review. German.
Cited by
-
Implanted islets in the anterior chamber of the eye are prone to autoimmune attack in a mouse model of diabetes.Diabetologia. 2013 Oct;56(10):2213-21. doi: 10.1007/s00125-013-3004-z. Epub 2013 Aug 11. Diabetologia. 2013. PMID: 23933952
-
Endocrine cell type sorting and mature architecture in the islets of Langerhans require expression of Roundabout receptors in β cells.Sci Rep. 2018 Jul 18;8(1):10876. doi: 10.1038/s41598-018-29118-x. Sci Rep. 2018. PMID: 30022126 Free PMC article.
-
Redox modulation protects islets from transplant-related injury.Diabetes. 2010 Jul;59(7):1731-8. doi: 10.2337/db09-0588. Epub 2010 Apr 22. Diabetes. 2010. PMID: 20413509 Free PMC article.
-
Determinants and dynamics of pancreatic islet architecture.Islets. 2022 Dec 31;14(1):82-100. doi: 10.1080/19382014.2022.2030649. Islets. 2022. PMID: 35258417 Free PMC article. Review.
-
A Collagen Based Cryogel Bioscaffold that Generates Oxygen for Islet Transplantation.Adv Funct Mater. 2020 Apr 14;30(15):1902463. doi: 10.1002/adfm.201902463. Epub 2020 Feb 20. Adv Funct Mater. 2020. PMID: 33071709 Free PMC article.
References
-
- Barshes NR, Wyllie S, Gross JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77:587–597. - PubMed
-
- Beger C, Cirulli V, Vajkoczy P, Halban PA, Menger MD. Vascularization of purified pancreatic islet-like cell aggregates (pseudoislet) after syngeneic transplantation. Diabetes. 1998;47:559–565. - PubMed
-
- Bretzel RG, Hering BJ, Schultz AO, Geier C, Federlin F. International islet transplant registry report. In: Lanza RP, Chick WL, editors. Yearbook of Cell and Tissue Transplantation. Dordrecht, the Netherlands: Kluwer Academic Publishers; 1996. pp. 153–160.
-
- Davalli AM, Ogawa Y, Ricordi C, Scharp DW, Bonner-Weir S, Weir GC. A selective decrease in the beta cell mass of human islets transplanted into diabetic nude mice. Transplantation. 1995;59:817–820. - PubMed
-
- Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of islet in the immediate posttransplantation period: dynamic changes in structure and function. Diabetes. 1996;45:1161–1167. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

