Less pain with Biatain-Ibu: initial findings from a randomised, controlled, double-blind clinical investigation on painful venous leg ulcers
- PMID: 17394627
- PMCID: PMC7951302
- DOI: 10.1111/j.1742-481X.2007.00312.x
Less pain with Biatain-Ibu: initial findings from a randomised, controlled, double-blind clinical investigation on painful venous leg ulcers
Abstract
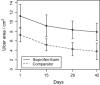
Six out of 10 patients with chronic wounds suffer from persistent wound pain. A multinational and multicentre, randomised, double-blind clinical investigation of 122 patients compared two moist wound-healing dressings, a non adhesive foam dressing with ibuprofen (62 patients randomised to Biatain-Ibu non adhesive, Coloplast A/S) with a non adhesive foam without ibuprofen (60 to Biatain non adhesive). The ibuprofen-foam was regarded successful, if the pain relief on a 5-point verbal rating scale was higher than the comparator without compromising safety, including appropriate healing rate. Additional endpoints were change in persistent wound pain between dressing changes and pain at dressing change on days 1-5 and days 43-47. The primary response variable, persistent pain relief, was significantly higher in the ibuprofen-foam group compared with the comparator on days 1-5, with a quick onset of action (P < 0.05). The patients in the ibuprofen-foam group had a significant (P < 0.05) higher reduction in the persistent wound pain from baseline (40%) as the comparator (30%). Women reported less pain intensity than men, and pain intensity decreased with increasing age. In addition, pain intensity increased with increasing initial pain intensity and increasing wound size. Wound healing was similar in the ibuprofen-foam group to that of the comparator group. No difference in adverse events between placebo and local sustained release of low-dose ibuprofen was observed in this study. This study has demonstrated that the ibuprofen-foam dressing provided pain relief and reduced pain intensity without compromising healing or other safety parameters. The full report of this study will be published in Wound Repair and Regeneration.
Conflict of interest statement
R. R. has acted as a paid researcher for Coloplast and received funding for that research. V. V. received funding for the salary of the research nurse involved in this work. P. V. and K. E. received renumeration for the extra time involved in study participation according to international guidelines. The authors are all members of the International Pain Advisory Board for Coloplast A/S and have been reimbursed for their services.
Figures



References
-
- Cooper SM, Hofman D, Burge SM. Leg ulcers and pain: a review. Int J Low Extrem Wounds 2003;2:189–97. - PubMed
-
- Hofman D, Ryan TJ, Arnold F, Cherry GW, Lindholm C, Bjellerup M, Glynn C. Pain in venous leg ulcers. J Wound Care 1997;6:222–4. - PubMed
-
- Phillips TJ, Stanton B, Provan A, Lew R. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol 1994;31:49–53. - PubMed
-
- Briggs M, Nelson EA. Topical agents or dressings for pain in venous leg ulcers. Cochrane Database Syst Rev 2005. (1): 23. - PubMed
-
- Flanagan M, Vogensen H, Haase L. Case series investigating the experience of pain in patients with chronic venous leg ulcers treated with a foam dressing releasing ibuprofen. World Wide Wounds 2006. [WWW document]. URL http://www.worldwidewounds.com/2006/april/Flanagan/Ibuprofen‐Foam‐Dressi... [accessed on 2 March 2007]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

