Regulation of MUC5AC expression by NAD(P)H:quinone oxidoreductase 1
- PMID: 17395013
- PMCID: PMC1913945
- DOI: 10.1016/j.freeradbiomed.2007.01.040
Regulation of MUC5AC expression by NAD(P)H:quinone oxidoreductase 1
Abstract
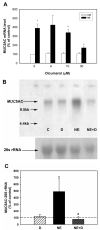
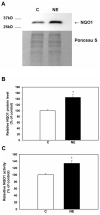
Neutrophil elastase (NE), a potent neutrophil inflammatory mediator, increases MUC5AC mucin gene expression through undefined pathways involving reactive oxygen species. To determine the source of NE-generated reactive oxygen species, we used pharmacologic inhibitors of oxidoreductases to test whether they blocked NE-regulated MUC5AC mRNA expression. We found that dicumarol, an inhibitor of the NADP(H):quinone oxidoreductase 1 (NQO1), inhibited MUC5AC mRNA expression in A549 lung adenocarcinoma cells and primary normal human bronchial epithelial cells. We further tested the role of NQO1 in mediating NE-induced MUC5AC expression by inhibiting NQO1 expression using short interfering RNA (siRNA). Transfection with siRNA specific for NQO1 suppressed NQO1 expression and significantly abrogated MUC5AC mRNA expression. NE treatment caused lipid peroxidation in A549 cells; this effect was inhibited by pretreatment with dicumarol, suggesting that NQO1 also regulates oxidant stress in A549 cells after NE exposure. NE exposure increased NQO1 protein and activity levels; NQO1 expression and activity were limited to the cytosol and did not translocate to the plasma membrane. Our results indicate that NQO1 has an important role as a key mediator of NE-regulated oxidant stress and MUC5AC mucin gene expression.
Figures






Similar articles
-
NAD(P)H quinone oxidoreductase 1 regulates neutrophil elastase-induced mucous cell metaplasia.Am J Physiol Lung Cell Mol Physiol. 2012 Aug 1;303(3):L181-8. doi: 10.1152/ajplung.00084.2012. Epub 2012 Jun 1. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22659878 Free PMC article.
-
Neutrophil elastase induces MUC5AC gene expression in airway epithelium via a pathway involving reactive oxygen species.Am J Respir Cell Mol Biol. 2002 Apr;26(4):447-52. doi: 10.1165/ajrcmb.26.4.4473. Am J Respir Cell Mol Biol. 2002. PMID: 11919081
-
Neutrophil elastase causes MUC5AC mucin synthesis via EGF receptor, ERK and NF-kB pathways in A549 cells.Korean J Intern Med. 2005 Dec;20(4):275-83. doi: 10.3904/kjim.2005.20.4.275. Korean J Intern Med. 2005. PMID: 16491824 Free PMC article.
-
Neutrophil elastase induces MUC5AC messenger RNA expression by an oxidant-dependent mechanism.Chest. 2000 May;117(5 Suppl 1):317S-20S. doi: 10.1378/chest.117.5_suppl_1.317s. Chest. 2000. PMID: 10843967
-
Interactions of the antioxidant enzymes NAD(P)H: Quinone oxidoreductase 1 (NQO1) and NRH: Quinone oxidoreductase 2 (NQO2) with pharmacological agents, endogenous biochemicals and environmental contaminants.Chem Biol Interact. 2021 Aug 25;345:109574. doi: 10.1016/j.cbi.2021.109574. Epub 2021 Jul 3. Chem Biol Interact. 2021. PMID: 34228969 Review.
Cited by
-
A candidate gene for autoimmune myasthenia gravis.Neurology. 2012 Jul 24;79(4):342-7. doi: 10.1212/WNL.0b013e318260cbd0. Epub 2012 Jun 27. Neurology. 2012. PMID: 22744667 Free PMC article.
-
NAD(P)H quinone oxidoreductase 1 regulates neutrophil elastase-induced mucous cell metaplasia.Am J Physiol Lung Cell Mol Physiol. 2012 Aug 1;303(3):L181-8. doi: 10.1152/ajplung.00084.2012. Epub 2012 Jun 1. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22659878 Free PMC article.
-
NADPH:quinone oxidoreductase 1 regulates host susceptibility to ozone via isoprostane generation.J Biol Chem. 2013 Feb 15;288(7):4681-91. doi: 10.1074/jbc.M112.438440. Epub 2012 Dec 28. J Biol Chem. 2013. PMID: 23275341 Free PMC article.
-
Neutrophil elastase increases airway epithelial nonheme iron levels.Clin Transl Sci. 2009 Oct;2(5):333-9. doi: 10.1111/j.1752-8062.2009.00151.x. Clin Transl Sci. 2009. PMID: 20411049 Free PMC article.
-
Neutrophil elastase activates macrophage calpain as a mechanism for phagocytic failure.Am J Physiol Lung Cell Mol Physiol. 2025 Jan 1;328(1):L93-L104. doi: 10.1152/ajplung.00132.2024. Epub 2024 Nov 5. Am J Physiol Lung Cell Mol Physiol. 2025. PMID: 39499256 Free PMC article.
References
-
- Welsh MJ, Ramsey BW, Accurso F, Cutting GR. Cystic fibrosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Diseases. III. McGraw-Hill; New York: 2001. pp. 5121–5188.
-
- Senior RM, Shapiro SD. Chronic obstructive pulmonary disease: epidemiology, pathophysiology, and pathogenesis. In: Fishman AP, editor. Fishman's Pulmonary Diseases and Disorders. Vol. 1. McGraw-Hill; New York: 1998. pp. 659–681.
-
- Voynow JA. What does mucin have to do with lung disease? Paediatr Respir Rev. 2002;3:98–103. - PubMed
-
- Konstan MW, Hilliard KA, Norvell TM, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am. J. Respir. Crit. Care Med. 1994;150:448–454. - PubMed
-
- Stockley RA, Burnett D. Alpha1-antitrypsin and leukocyte elastase in infected and noninfected sputum. Am Rev Respir Dis. 1979;120:1081–1086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

