Many NK cell receptors activate ERK2 and JNK1 to trigger microtubule organizing center and granule polarization and cytotoxicity
- PMID: 17395718
- PMCID: PMC1851054
- DOI: 10.1073/pnas.0611655104
Many NK cell receptors activate ERK2 and JNK1 to trigger microtubule organizing center and granule polarization and cytotoxicity
Abstract
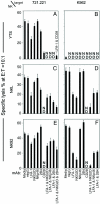
Natural killer (NK) cells are components of the innate immune system that recognize and kill tumor or virus-infected target cells through specific NK activating receptor/ligand interactions. Lymphocyte function-associated antigen (LFA)-1 and its ligand ICAM-1 are also required to initiate conjugation and actin cytoskeletal remodeling. The NK activating receptors, many of which are expressed on a single NK cell, signal the polarization of the microtubule organizing center (MTOC) together with cytolytic granules to the synapse with target cells. After ligation of any one of these receptors, Src family kinases initiate activation of two signal pathways, the phosphoinositide-3 kinase --> ERK2 and the phospholipase Cgamma --> JNK1 pathways. Both are required for polarization of the MTOC and cytolytic granules, a prerequisite for killing the targets. Crosslinking of CD28, NKG2D, NKp30, NKp46, NKG2C/CD94, or 2B4 leads to the phosphorylation of both ERK2 and JNK1, although they use different proximal signaling modules. Thus, many, if not all, activating receptors stimulate these two distal pathways, independent of the proximal signaling module used. By contrast, CD2, DNAM-1, and beta(1)-integrin crosslinking do not activate either pathway; they may be costimulatory molecules or have another function in the synapse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells.Proc Natl Acad Sci U S A. 2006 Jul 5;103(27):10346-10351. doi: 10.1073/pnas.0604236103. Epub 2006 Jun 26. Proc Natl Acad Sci U S A. 2006. PMID: 16801532 Free PMC article.
-
JNK MAP kinase activation is required for MTOC and granule polarization in NKG2D-mediated NK cell cytotoxicity.Proc Natl Acad Sci U S A. 2008 Feb 26;105(8):3017-22. doi: 10.1073/pnas.0712310105. Epub 2008 Feb 19. Proc Natl Acad Sci U S A. 2008. PMID: 18287025 Free PMC article.
-
Rapid activation receptor- or IL-2-induced lytic granule convergence in human natural killer cells requires Src, but not downstream signaling.Blood. 2013 Apr 4;121(14):2627-37. doi: 10.1182/blood-2012-06-437012. Epub 2013 Feb 4. Blood. 2013. PMID: 23380740 Free PMC article.
-
Molecular regulation of the plasma membrane-proximal cellular steps involved in NK cell cytolytic function.J Cell Sci. 2020 Feb 21;133(5):jcs240424. doi: 10.1242/jcs.240424. J Cell Sci. 2020. PMID: 32086255 Free PMC article. Review.
-
Regulation of self-ligands for activating natural killer cell receptors.Ann Med. 2013 Jun;45(4):384-94. doi: 10.3109/07853890.2013.792495. Epub 2013 May 23. Ann Med. 2013. PMID: 23701136 Review.
Cited by
-
Functional Alteration of Natural Killer Cells and Cytotoxic T Lymphocytes upon Asbestos Exposure and in Malignant Mesothelioma Patients.Biomed Res Int. 2015;2015:238431. doi: 10.1155/2015/238431. Epub 2015 Jun 16. Biomed Res Int. 2015. PMID: 26161391 Free PMC article. Review.
-
Engagement of TRAIL triggers degranulation and IFNγ production in human natural killer cells.EMBO Rep. 2022 Aug 3;23(8):e54133. doi: 10.15252/embr.202154133. Epub 2022 Jun 27. EMBO Rep. 2022. PMID: 35758160 Free PMC article.
-
LFA-1 regulates CD8+ T cell activation via T cell receptor-mediated and LFA-1-mediated Erk1/2 signal pathways.J Biol Chem. 2009 Jul 31;284(31):21001-10. doi: 10.1074/jbc.M109.002865. Epub 2009 May 29. J Biol Chem. 2009. PMID: 19483086 Free PMC article.
-
Celecoxib increases lung cancer cell lysis by lymphokine-activated killer cells via upregulation of ICAM-1.Oncotarget. 2015 Nov 17;6(36):39342-56. doi: 10.18632/oncotarget.5745. Oncotarget. 2015. PMID: 26513172 Free PMC article.
-
Coordinated Activation of Toll-Like Receptor8 (TLR8) and NLRP3 by the TLR8 Agonist, VTX-2337, Ignites Tumoricidal Natural Killer Cell Activity.PLoS One. 2016 Feb 29;11(2):e0148764. doi: 10.1371/journal.pone.0148764. eCollection 2016. PLoS One. 2016. PMID: 26928328 Free PMC article.
References
-
- Cerwenka A, Lanier LL. Nat Rev Immunol. 2001;1:41–49. - PubMed
-
- Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. Immunity. 2002;17:19–29. - PubMed
-
- Chiesa S, Tomasello E, Vivier E, Vely F. Mol Immunol. 2005;42:477–484. - PubMed
-
- Eissmann P, Beauchamp L, Wooters J, Tilton JC, Long EO, Watzl C. Blood. 2005;105:4722–4729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

