Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells
- PMID: 17395742
- PMCID: PMC3746188
- DOI: 10.2337/db06-1254
Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells
Abstract
Endothelial precursor cells (EPCs) play a key role in vascular repair and maintenance, and their function is impeded in diabetes. We previously demonstrated that EPCs isolated from diabetic patients have a profound inability to migrate in vitro. We asked whether EPCs from normal individuals are better able to repopulate degenerate (acellular) retinal capillaries in chronic (diabetes) and acute (ischemia/reperfusion [I/R] injury and neonatal oxygen-induced retinopathy [OIR]) animal models of ocular vascular damage. Streptozotocin-induced diabetic mice, spontaneously diabetic BBZDR/Wor rats, adult mice with I/R injury, or neonatal mice with OIR were injected within the vitreous or the systemic circulation with fluorescently labeled CD34(+) cells from either diabetic patients or age- and sex-matched healthy control subjects. At specific times after administering the cells, the degree of vascular repair of the acellular capillaries was evaluated immunohistologically and quantitated. In all four models, healthy human (hu)CD34(+) cells attached and assimilated into vasculature, whereas cells from diabetic donors uniformly were unable to integrate into damaged vasculature. These studies demonstrate that healthy huCD34(+) cells can effectively repair injured retina and that there is defective repair of vasculature in patients with diabetes. Defective EPCs may be amenable to pharmacological manipulation and restoration of the cells' natural robust reparative function.
Figures
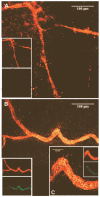



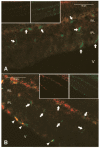
References
-
- Fadini GP, Sartore S, Baesso I, Lenzi M, Agostini C, Tiengo A, Avogaro A. Endothelial progenitor cells and the diabetic paradox. Diabetes Care. 2006;29:714–716. - PubMed
-
- Awad O, Dedkov E, Jiao C, Bloomer S, Tomanek RJ, Schatteman GC. Differential healing activities of CD34+ and CD14+ endothelial cell progenitors. Arterioscler Thromb Vasc Biol. 2006;26:758–764. - PubMed
-
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. - PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

