Enteroendocrine cells express functional Toll-like receptors
- PMID: 17395901
- PMCID: PMC3203538
- DOI: 10.1152/ajpgi.00249.2006
Enteroendocrine cells express functional Toll-like receptors
Abstract
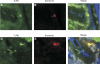
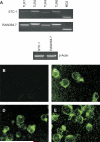
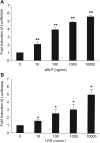
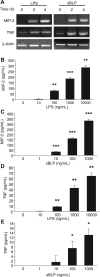
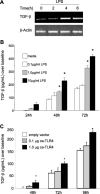
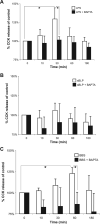
Intestinal epithelial cells (IECs) provide a physical and immunological barrier against enteric microbial flora. Toll-like receptors (TLRs), through interactions with conserved microbial patterns, activate inflammatory gene expression in cells of the innate immune system. Previous studies of the expression and function of TLRs in IECs have reported varying results. Therefore, TLR expression was characterized in human and murine intestinal sections, and TLR function was tested in an IEC line. TLR1, TLR2, and TLR4 are coexpressed on a subpopulation of human and murine IECs that reside predominantly in the intestinal crypt and belong to the enteroendocrine lineage. An enteroendocrine cell (EEC) line demonstrated a similar expression pattern of TLRs as primary cells. The murine EEC line STC-1 was activated with specific TLR ligands: LPS or synthetic bacterial lipoprotein. In STC-1 cells stimulated with bacterial ligands, NF-kappaB and MAPK activation was demonstrated. Furthermore, the expression of TNF and macrophage inhibitory protein-2 were induced. Additionally, bacterial ligands induced the expression of the anti-inflammatory gene transforming growth factor-beta. LPS triggered a calcium flux in STC-1 cells, resulting in a rapid increase in CCK secretion. Finally, conditioned media from STC-1 cells inhibited the production of nitric oxide and IL-12 p40 by activated macrophages. In conclusion, human and murine IECs that express TLRs belong to the enteroendocrine lineage. Using a murine EEC model, a broad range of functional effects of TLR activation was demonstrated. This study suggests a potential role for EECs in innate immune responses.
Figures







References
-
- Abad C, Martinez C, Juarranz MG, Arranz A, Leceta J, Delgado M, Gomariz RP. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology. 2003;124:961–971. - PubMed
-
- Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167:1609–1616. - PubMed
-
- Barg S. Mechanisms of exocytosis in insulin-secreting B-cells and glucagon-secreting A-cells. Pharmacol Toxicol. 2003;92:3–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

