A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea
- PMID: 17396154
- PMCID: PMC1852784
- DOI: 10.1038/sj.emboj.7601658
A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea
Abstract
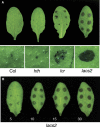
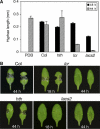
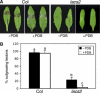
The plant cuticle composed of cutin, a lipid-derived polyester, and cuticular waxes covers the aerial portions of plants and constitutes a hydrophobic extracellular matrix layer that protects plants against environmental stresses. The botrytis-resistant 1 (bre1) mutant of Arabidopsis reveals that a permeable cuticle does not facilitate the entry of fungal pathogens in general, but surprisingly causes an arrest of invasion by Botrytis. BRE1 was identified to be long-chain acyl-CoA synthetase2 (LACS2) that has previously been shown to be involved in cuticle development and was here found to be essential for cutin biosynthesis. bre1/lacs2 has a five-fold reduction in dicarboxylic acids, the typical monomers of Arabidopsis cutin. Comparison of bre1/lacs2 with the mutants lacerata and hothead revealed that an increased permeability of the cuticle facilitates perception of putative elicitors in potato dextrose broth, leading to the presence of antifungal compound(s) at the surface of Arabidopsis plants that confer resistance to Botrytis and Sclerotinia. Arabidopsis plants with a permeable cuticle have thus an altered perception of their environment and change their physiology accordingly.
Figures
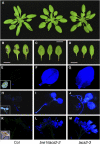
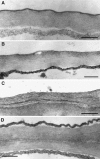
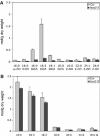
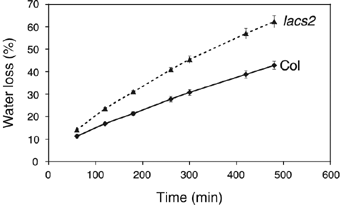


References
-
- Benveniste I, Tijet N, Adas F, Philipps G, Salaun JP, Durst F (1998) CYP86A1 from Arabidopsis thaliana encodes a cytochrome P450-dependent fatty acid omega-hydroxylase. Biochem Biophys Res Commun 243: 688–693 - PubMed
-
- Berrocal-Lobo M, Molina A, Solano R (2002) Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J 29: 23–32 - PubMed
-
- Bonaventure G, Beisson F, Ohlrogge J, Pollard M (2004) Analysis of the aliphatic monomer composition of polyesters associated with Arabidopsis epidermis: occurrence of octadeca-cis-6, cis-9-diene-1,18-dioate as the major component. Plant J 49: 920–930 - PubMed
-
- Burghardt M, Riederer M (2006) Cuticular transpiration. In Biology of the Plant Cuticle, Riederer M, Müller C (eds) pp 292–311. Oxford: Blackwell Publishing
-
- Chassot C, Métraux JP (2005) The cuticle as source of signals for plant defense. Plant Biosystems 139: 28–31
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

