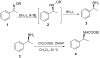
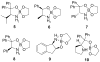Asymmetric synthesis of primary amines via the spiroborate-catalyzed borane reduction of oxime ethers
- PMID: 17397177
- PMCID: PMC2571073
- DOI: 10.1021/ol0704791
Asymmetric synthesis of primary amines via the spiroborate-catalyzed borane reduction of oxime ethers
Abstract
[reaction: see text] The enantioselective borane reduction of O-benzyloxime ethers to primary amines was studied under catalytic conditions using the spiroborate esters 5-10 derived from nonracemic 1,2-amino alcohols and ethylene glycol. Effective catalytic conditions were achieved using only 10% of catalyst 5 derived from diphenylvalinol in dioxane at 0 degrees C resulting in complete conversion to the corresponding primary amine in up to 99% ee.
Figures
References
-
- Johansson A. Contemp Org Synth. 1995;266:393–406. and ref. cited therein.
- Deloux L, Srebnik M. Chem Rev. 1993;93:763–784.
-
- Glushkov VA, Tolstikov AG. Russ Chem Rev (Engl Transl) 2004;73:581–608. and ref. cited therein.
- Cho BT. Tetrahedron. 2006;62:7621–7643. and ref. cited therein.
- Brown JM, Guy C, Lloyd-Jones GC, Layzell TP. Tetrahedron: Asymmetry. 1993;4:2151–2154.
- Lang A, Noth H, Schmidt M. Chem Ber. 1997;130:241–246.
- Mathre DJ, Thompson AS, Douglas AW, Carroll JD, Corley EG, Grabowski EJJ. J Org Chem. 1993;58:2880–2888.
-
- Itsuno S, Nakano M, Miyazaki K, Masuda H, Ito K. J Chem Soc Perkin Tran 1. 1985:2039–2044.
- Itsuno S, Sakurai Y, Ito K, Hirao A, Nakahama S. Bull Chem Soc Jpn. 1987;60:395–396.
- Itsuno S, Sakurai Y, Shimizu K, Ito K. J Chem Soc Perkin Trans 1. 1990:1859–1863.
- Bolm C, Felder M. Synlett. 1994:655–666.
- Cho BT, Ryu MH. Bull Korean Chem Soc. 1994;15:191–192.
- Lantos I, Flisak J, Liu L, Matsunoka R, Mendelson W, Stevenson D, Tubman K, Tucker L, Zhang WY, Adams J, Sorenson M, Garigipati R, Erhardt K, Ross S. J Org Chem. 1997;62:5385–5391.
- Demir AS. Pure Appl Chem. 1997;69:105–108.
- Inoue T, Sato D, Komura K, Itsuno S. Tetrahedron Lett. 1999;40:5379–5382.
- Itsuno S, Matsumoto T, Sato D, Inoue T. J Org Chem. 2000;65:5879–5881. - PubMed
- Sailes HE, Watts JP, Whiting A. J Chem Soc Perkin Trans. 1;2000:3362–3374.
- Fontaine E, Namane C, Meneyrol J, Geslin M, Serva L, Roussey E, Tissandié S, Maftouh M, Roger P. Tetrahedron: Asymmetry. 2001;12:2185–2189.
- Krzeminski MP, Zaidlewicz M. Tetrahedron: Asymmetry. 2003;14:1463–1466.
- Sakito Y, Yoneyoshi Y, Suzukamo G. Tetrahedron Lett. 1988;29:223–224.
-
- Tillyer RD, Boudreau C, Tschaen D, Dolling U-H, Reider PJ. Tetrahedron Lett. 1995;36:4337–4340.
- Shimizu M, Kamei M, Fujisawa T. Tetrahedron Lett. 1995;36:8607–8610.
- Shimizu M, Tsukamoto K, Matsutani T, Fujisawa T. Tetrahedron. 1998;54:10265–10274.
- Masui M, Shioiri T. Tetrahedron Lett. 1998;39:5195–5198.
-
- Chu YB, Shan ZX, Liu DJ, Sun NN. J Org Chem. 2006;71:3998–4001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources