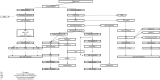
Modern plasma fractionation
- PMID: 17397761
- PMCID: PMC7125842
- DOI: 10.1016/j.tmrv.2006.11.001
Modern plasma fractionation
Abstract
Protein products fractionated from human plasma are an essential class of therapeutics used, often as the only available option, in the prevention, management, and treatment of life-threatening conditions resulting from trauma, congenital deficiencies, immunologic disorders, or infections. Modern plasma product production technology remains largely based on the ethanol fractionation process, but much has evolved in the last few years to improve product purity, to enhance the recovery of immunoglobulin G, and to isolate new plasma proteins, such as alpha1-protease inhibitor, von Willebrand factor, and protein C. Because of the human origin of the starting material and the pooling of 10,000 to 50,000 donations required for industrial processing, the major risk associated to plasma products is the transmission of blood-borne infectious agents. A complete set of measures--and, most particularly, the use of dedicated viral inactivation and removal treatments--has been implemented throughout the production chain of fractionated plasma products over the last 20 years to ensure optimal safety, in particular, and not exclusively, against HIV, hepatitis B virus, and hepatitis C virus. In this review, we summarize the practices of the modern plasma fractionation industry from the collection of the raw plasma material to the industrial manufacture of fractionated products. We describe the quality requirements of plasma for fractionation and the various treatments applied for the inactivation and removal of blood-borne infectious agents and provide examples of methods used for the purification of the various classes of plasma protein therapies. We also highlight aspects of the good manufacturing practices and the regulatory environment that govern the whole chain of production. In a regulated and professional environment, fractionated plasma products manufactured by modern processes are certainly among the lowest-risk therapeutic biological products in use today.
Figures
References
-
- WHO Recommendations for the production, quality control and regulation of plasma for fractionation. 2005. http://www.who.int/bloodproducts
-
- CHMP: Guideline on epidemiological data on blood transmissible infections. For inclusion in the Guideline on the Scientific data requirements for a Plasma Master File. (EMEA/CPMP/BWP/3794/03). EMEA/CPMP/BWP/125/04, January 2005. http://www.emea.eu.int: European Medicine Agency, 2005.
-
- Burgstaler E. Current instrumentation for apheresis. In: McLeod B.C., Price T.H., Weinstein R., editors. Apheresis: Principles and practice. ed 2. AABB Press; Bethesda, MD: 2003. pp. 95–130.
-
- Burnouf T., Kappelsberger C., Frank K. Residual cell content in plasma from 3 centrifugal apheresis procedures. Transfusion. 2003;11:1522–1526. - PubMed
-
- Sonntag J., Emeis M., Vornwald A. Complement activation during plasma production depends on the apheresis technique. Transfus Med. 1998;8:205–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical