Virus glycosylation: role in virulence and immune interactions
- PMID: 17398101
- PMCID: PMC7127133
- DOI: 10.1016/j.tim.2007.03.003
Virus glycosylation: role in virulence and immune interactions
Abstract
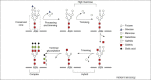
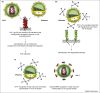
The study of N-linked glycosylation as it relates to virus biology has become an area of intense interest in recent years due to its ability to impart various advantages to virus survival and virulence. HIV and influenza, two clear threats to human health, have been shown to rely on expression of specific oligosaccharides to evade detection by the host immune system. Additionally, other viruses such as Hendra, SARS-CoV, influenza, hepatitis and West Nile rely on N-linked glycosylation for crucial functions such as entry into host cells, proteolytic processing and protein trafficking. This review focuses on recent findings on the importance of glycosylation to viral virulence and immune evasion for several prominent human pathogens.
Figures
References
-
- Land A., Braakman I. Folding of the human immunodeficiency virus type 1 envelope glycoprotein in the endoplasmic reticulum. Biochimie. 2001;83:783–790. - PubMed
-
- Slater-Handshy T. HCV E2 glycoprotein: mutagenesis of N-linked glycosylation sites and its effects on E2 expression and processing. Virology. 2004;319:36–48. - PubMed
-
- Meunier J.C. Analysis of the glycosylation sites of hepatitis C virus (HCV) glycoprotein E1 and the influence of E1 glycans on the formation of the HCV glycoprotein complex. J. Gen. Virol. 1999;80:887–896. - PubMed
-
- Klenk H.D. Importance of hemagglutinin glycosylation for the biological functions of influenza virus. Virus Res. 2002;82:73–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

