Assay of mucins in human tear fluid
- PMID: 17399701
- PMCID: PMC1950265
- DOI: 10.1016/j.exer.2007.01.018
Assay of mucins in human tear fluid
Abstract
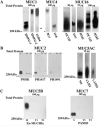
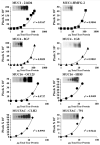
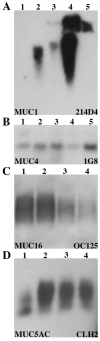
Mucin genes, both secreted (MUC2, MUC5AC, MUC5B, MUC7) and membrane associated (MUC1, MUC4, MUC16), have been reported to be expressed by ocular surface epithelia. The purpose of this study was to comprehensively assay the mucin content of human tear fluid using multiple antibodies for each mucin and to develop a sensitive, semi-quantitative method for the assay of mucins in tears. Tear washes were obtained by instillation of saline onto the ocular surface, followed by collection from the inferior fornix. Tear proteins were separated in 1% agarose gels, transferred to nitrocellulose membrane by vacuum blotting and probed with multiple antibodies recognizing MUC1, MUC2, MUC4, MUC5AC, MUC5B, MUC7 and MUC16. Binding was detected using chemiluminescence, and quantity was determined by densitometry. Serial dilutions of pooled tears from normal individuals were assayed to determine the linear range of detectability. MUC1, MUC4, MUC16, MUC5AC and low levels of MUC2 were consistently detected in human tear fluid, while MUC5B and MUC7 were not. Use of several antibodies recognizing different epitopes on the same mucin confirmed these findings. The antibodies to mucins bound to serial dilutions of tears in a linear fashion (r2 > 0.9), indicating the feasibility of semi-quantitation. MUC5AC in tear fluid had an increased electrophoretic mobility compared to MUC5AC isolated from conjunctival tissue. This study provides clear evidence that the mucin component of tears is a mixture of secreted and shed membrane-associated mucins, and for the first time demonstrates MUC16 in tear fluid. Immunoblots of tears using agarose gel electrophoresis and chemiluminescence detection provide a semi-quantitative assay for mucin protein that will be useful for comparisons with tears from diseased eyes or after pharmacological intervention.
Figures
Similar articles
-
Concentrations of MUC16 and MUC5AC using three tear collection methods.Mol Vis. 2017 Jul 28;23:529-537. eCollection 2017. Mol Vis. 2017. PMID: 28761326 Free PMC article.
-
Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjögren syndrome.Invest Ophthalmol Vis Sci. 2002 Apr;43(4):1004-11. Invest Ophthalmol Vis Sci. 2002. PMID: 11923240
-
Expression of membrane-bound mucins (MUC1 and MUC4) and secreted mucins (MUC2, MUC5AC, MUC5B, MUC6 and MUC7) in mucoepidermoid carcinomas of salivary glands.Am J Surg Pathol. 2005 Jun;29(6):806-13. doi: 10.1097/01.pas.0000155856.84553.c9. Am J Surg Pathol. 2005. PMID: 15897748
-
Ocular Surface Membrane-Associated Mucins.Ocul Surf. 2016 Jul;14(3):331-41. doi: 10.1016/j.jtos.2016.03.003. Epub 2016 May 3. Ocul Surf. 2016. PMID: 27154035 Review.
-
Distribution of mucins at the ocular surface.Exp Eye Res. 2004 Mar;78(3):379-88. doi: 10.1016/s0014-4835(03)00204-5. Exp Eye Res. 2004. PMID: 15106916 Review.
Cited by
-
Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function.PLoS One. 2014 Jun 26;9(6):e100393. doi: 10.1371/journal.pone.0100393. eCollection 2014. PLoS One. 2014. PMID: 24968021 Free PMC article.
-
Generation and characterization of a monoclonal antibody to the cytoplasmic tail of MUC16.Glycobiology. 2017 Oct 1;27(10):920-926. doi: 10.1093/glycob/cwx054. Glycobiology. 2017. PMID: 28673046 Free PMC article.
-
Comparison of mucin levels at the ocular surface of postmenopausal women with and without a history of dry eye.Cornea. 2011 Dec;30(12):1346-52. doi: 10.1097/ICO.0b013e31820d852a. Cornea. 2011. PMID: 22089171 Free PMC article.
-
Glycobiology of the ocular surface: mucins and lectins.Jpn J Ophthalmol. 2013 Mar;57(2):150-5. doi: 10.1007/s10384-012-0228-2. Epub 2013 Jan 17. Jpn J Ophthalmol. 2013. PMID: 23325272 Free PMC article. Review.
-
Regional Comparison of Goblet Cell Number and Area in Exposed and Covered Dry Eyes and Their Correlation with Tear MUC5AC.Sci Rep. 2020 Feb 19;10(1):2933. doi: 10.1038/s41598-020-59956-7. Sci Rep. 2020. PMID: 32076085 Free PMC article.
References
-
- Akagi J, Takai E, Tamori Y, Nakagawa K, Ogawa M. CA19-9 epitope a possible marker for MUC-1/Y protein. Int J Oncol. 2001;18:1085–1091. - PubMed
-
- Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren’s syndrome. Invest Ophthalmol Vis Sci. 2002;43:1004–1011. - PubMed
-
- Argueso P, Herreras J, Calonge M, Citores L, Pastor J, Girbes T. Analysis of human ocular mucus: effects of neuraminidase and chitinase enzymes. Cornea. 1998;17:200–207. - PubMed
-
- Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 2003a;44:2487–2495. - PubMed
-
- Argueso P, Tisdale A, Mandel U, Letko E, Foster CS, Gipson IK. The cell-layer- and cell-type-specific distribution of GalNAc-transferases in the ocular surface epithelia is altered during keratinization. Invest Ophthalmol Vis Sci. 2003b;44:86–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

