Crystal structures of a quorum-quenching antibody
- PMID: 17400249
- PMCID: PMC1994716
- DOI: 10.1016/j.jmb.2007.02.081
Crystal structures of a quorum-quenching antibody
Abstract
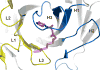
A large number of Gram-negative bacteria employ N-acyl homoserine lactones (AHLs) as signaling molecules in quorum sensing, which is a population density-dependent mechanism to coordinate gene expression. Antibody RS2-1G9 was elicited against a lactam mimetic of the N-acyl homoserine lactone and represents the only reported monoclonal antibody that recognizes the naturally-occuring N-acyl homoserine lactone with high affinity. Due to its high cross-reactivity, RS2-1G9 showed remarkable inhibition of quorum sensing signaling in Pseudomonas aeruginosa, a common opportunistic pathogen in humans. The crystal structure of Fab RS2-1G9 in complex with a lactam analog revealed complete encapsulation of the polar lactam moiety in the antibody-combining site. This mode of recognition provides an elegant immunological solution for tight binding to an aliphatic, lipid-like ligand with a small head group lacking typical haptenic features, such as aromaticity or charge, which are often incorporated into hapten design to generate high-affinity antibodies. The ability of RS2-1G9 to discriminate between closely related AHLs is conferred by six hydrogen bonds to the ligand. Conversely, cross-reactivity of RS2-1G9 towards the lactone is likely to originate from conservation of these hydrogen bonds as well as an additional hydrogen bond to the oxygen of the lactone ring. A short, narrow tunnel exiting at the protein surface harbors a portion of the acyl chain and would not allow entry of the head group. The crystal structure of the antibody without its cognate lactam or lactone ligands revealed a considerably altered antibody-combining site with a closed binding pocket. Curiously, a completely buried ethylene glycol molecule mimics the lactam ring and, thus, serves as a surrogate ligand. The detailed structural delineation of this quorum-quenching antibody will aid further development of an antibody-based therapy against bacterial pathogens by interference with quorum sensing.
Figures






Similar articles
-
The quorum quenching antibody RS2-1G9 protects macrophages from the cytotoxic effects of the Pseudomonas aeruginosa quorum sensing signalling molecule N-3-oxo-dodecanoyl-homoserine lactone.Mol Immunol. 2008 May;45(9):2710-4. doi: 10.1016/j.molimm.2008.01.010. Epub 2008 Mar 4. Mol Immunol. 2008. PMID: 18304641 Free PMC article.
-
Quorum quenching by an N-acyl-homoserine lactone acylase from Pseudomonas aeruginosa PAO1.Infect Immun. 2006 Mar;74(3):1673-82. doi: 10.1128/IAI.74.3.1673-1682.2006. Infect Immun. 2006. PMID: 16495538 Free PMC article.
-
Structural determinants driving homoserine lactone ligand selection in the Pseudomonas aeruginosa LasR quorum-sensing receptor.Proc Natl Acad Sci U S A. 2019 Jan 2;116(1):245-254. doi: 10.1073/pnas.1817239116. Epub 2018 Dec 17. Proc Natl Acad Sci U S A. 2019. PMID: 30559209 Free PMC article.
-
Heterocyclic Chemistry Applied to the Design of N-Acyl Homoserine Lactone Analogues as Bacterial Quorum Sensing Signals Mimics.Molecules. 2021 Aug 24;26(17):5135. doi: 10.3390/molecules26175135. Molecules. 2021. PMID: 34500565 Free PMC article. Review.
-
Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms.Proc Natl Acad Sci U S A. 2000 Aug 1;97(16):8789-93. doi: 10.1073/pnas.97.16.8789. Proc Natl Acad Sci U S A. 2000. PMID: 10922036 Free PMC article. Review.
Cited by
-
Quorum sensing: A nobel target for antibacterial agents.Avicenna J Med. 2012 Oct;2(4):97-9. doi: 10.4103/2231-0770.110743. Avicenna J Med. 2012. PMID: 23826557 Free PMC article. No abstract available.
-
Structural basis for ligand recognition and discrimination of a quorum-quenching antibody.J Biol Chem. 2011 May 13;286(19):17351-8. doi: 10.1074/jbc.M111.231258. Epub 2011 Mar 23. J Biol Chem. 2011. PMID: 21454495 Free PMC article.
-
Composition, anti-quorum sensing and antimicrobial activity of essential oils from Lippia alba.Braz J Microbiol. 2014 Oct 9;45(3):759-67. doi: 10.1590/s1517-83822014000300001. eCollection 2014. Braz J Microbiol. 2014. PMID: 25477905 Free PMC article.
-
Plant-made antibody against miroestrol: a new platform for expression of full-length immunoglobulin G against small-molecule targets in immunoassays.Plant Cell Rep. 2021 Apr;40(4):723-733. doi: 10.1007/s00299-021-02670-z. Epub 2021 Feb 13. Plant Cell Rep. 2021. PMID: 33582859
-
Direct Quantitative Immunochemical Analysis of Autoinducer Peptide IV for Diagnosing and Stratifying Staphylococcus aureus Infections.ACS Infect Dis. 2022 Mar 11;8(3):645-656. doi: 10.1021/acsinfecdis.1c00670. Epub 2022 Feb 17. ACS Infect Dis. 2022. PMID: 35175740 Free PMC article.
References
-
- Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439–468. - PubMed
-
- Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. - PubMed
-
- Smith RS, Iglewski BH. P. aeruginosa quorum-sensing systems and virulence. Curr Opin Microbiol. 2003;6:56–60. - PubMed
-
- Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, Wood MR, Brogan AP, Lehmann M, Mee JM, Iwata K, Pan Q, Fearns C, Knaus UG, Meijler MM, Janda KD, Ulevitch RJ. N-(3-oxo-acyl)homoserine lactones signal cell activation through a mechanism distinct from the canonical pathogen-associated molecular pattern recognition receptor pathways. J Biol Chem. 2006;281:28822–28830. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

