Cycloheximide: no ordinary bitter stimulus
- PMID: 17400304
- PMCID: PMC1995601
- DOI: 10.1016/j.bbr.2007.02.027
Cycloheximide: no ordinary bitter stimulus
Abstract
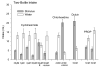
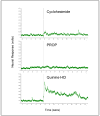
Cycloheximide (CyX), a toxic antibiotic with a unique chemical structure generated by the actinomycete, Streptomyces griseus, has emerged as a primary focus of studies on mammalian bitter taste. Rats and mice avoid it at concentrations well below the thresholds for most bitter stimuli and T2R G-protein-coupled receptors specific for CyX with appropriate sensitivity are identified for those species. Like mouse and rat, golden hamsters, Mesocricetus auratus, also detected and rejected micromolar levels of CyX, although 1mM CyX failed to activate the hamster chorda tympani nerve. Hamsters showed an initial tolerance for 500microM CyX, but after that, avoidance of CyX dramatically increased, plasticity not reported for rat or mouse. As the hamster lineage branches well before division of the mouse-rat lineage in evolutionary time, differences between hamster and mouse-rat reactions to CyX are not surprising. Furthermore, unlike hamster LiCl-induced learned aversions, the induced CyX aversion neither specifically nor robustly generalized to other non-ionic bitter stimuli; and unlike adverse reactions to other chemosensory stimuli, aversions to CyX were not mollified by adding a sweetener. Thus, CyX is unlike other bitter stimuli. The gene for the high-affinity CyX receptor is a member of a cluster of five orthologous T2R genes that are likely rodent-specific; this "CyX clade" is found in the mouse, rat and probably hamster, but not in the human or rabbit genome. The rodent CyX-T2R interaction may be one of multiple lineage-specific stimulus-receptor interactions reflecting a response to a particular environmental toxin. The combination of T2R multiplicity, species divergence and gene duplication results in diverse ligands for multiple species-specific T2R receptors, which confounds definition of 'bitter' stimuli across species.
Figures






Similar articles
-
The distinctiveness of ionic and nonionic bitter stimuli.Physiol Behav. 2004 Jan;80(4):421-31. doi: 10.1016/j.physbeh.2003.09.009. Physiol Behav. 2004. PMID: 14741226
-
Analysis of polysaccharide taste in hamsters: behavioral and neural studies.Physiol Behav. 1996 Mar;59(3):505-16. doi: 10.1016/0031-9384(95)02092-6. Physiol Behav. 1996. PMID: 8700954
-
Loss and recovery of sodium-salt taste following bilateral chorda tympani nerve crush.Physiol Behav. 1993 Jan;53(1):75-80. doi: 10.1016/0031-9384(93)90013-6. Physiol Behav. 1993. PMID: 8434072
-
Chorda tympani responses in two inbred strains of mice with different taste preferences.Physiol Behav. 1999 Aug;67(2):287-97. doi: 10.1016/s0031-9384(99)00071-2. Physiol Behav. 1999. PMID: 10477061 Review.
-
Neuron types, receptors, behavior, and taste quality.Physiol Behav. 2000 Apr 1-15;69(1-2):53-62. doi: 10.1016/s0031-9384(00)00188-8. Physiol Behav. 2000. PMID: 10854917 Review.
Cited by
-
Glossopharyngeal nerve transection impairs unconditioned avoidance of diverse bitter stimuli in rats.Behav Neurosci. 2011 Aug;125(4):519-28. doi: 10.1037/a0023934. Behav Neurosci. 2011. PMID: 21604835 Free PMC article.
-
Taste coding after selective inhibition by chlorhexidine.Chem Senses. 2009 Oct;34(8):653-66. doi: 10.1093/chemse/bjp047. Epub 2009 Aug 24. Chem Senses. 2009. PMID: 19703921 Free PMC article.
-
Novel, Fully Characterised Bovine Taste Bud Cells of Fungiform Papillae.Cells. 2021 Sep 2;10(9):2285. doi: 10.3390/cells10092285. Cells. 2021. PMID: 34571933 Free PMC article.
-
Salt taste inhibition by cathodal current.Brain Res Bull. 2009 Sep 28;80(3):107-15. doi: 10.1016/j.brainresbull.2009.06.019. Epub 2009 Jul 1. Brain Res Bull. 2009. PMID: 19576268 Free PMC article.
-
Cracking taste codes by tapping into sensory neuron impulse traffic.Prog Neurobiol. 2008 Nov;86(3):245-63. doi: 10.1016/j.pneurobio.2008.09.003. Epub 2008 Sep 7. Prog Neurobiol. 2008. PMID: 18824076 Free PMC article. Review.
References
-
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJP, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. - PubMed
-
- Araneda RC, Kini AD, Firestein SK. The molecular receptive range of an odorant receptor. Nat Neurosci. 2000;3:1248–1255. - PubMed
-
- Arvola A, Forsander O. Comparison between water and alcohol consumption in six animal species in free-choice experiments. Nature. 1961;191:819–820. - PubMed
-
- Beidler LM, Fishman IY, Hardiman CW. Species differences in taste responses. Am J Physiol. 1955;181:235–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

