PsrA, the Pseudomonas sigma regulator, controls regulators of epiphytic fitness, quorum-sensing signals, and plant interactions in Pseudomonas syringae pv. tomato strain DC3000
- PMID: 17400767
- PMCID: PMC1932703
- DOI: 10.1128/AEM.02445-06
PsrA, the Pseudomonas sigma regulator, controls regulators of epiphytic fitness, quorum-sensing signals, and plant interactions in Pseudomonas syringae pv. tomato strain DC3000
Abstract
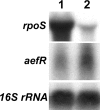
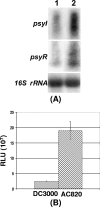
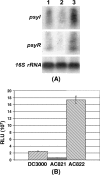
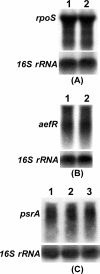
Pseudomonas syringae pv. tomato strain DC3000, a pathogen of tomato and Arabidopsis, occurs as an epiphyte. It produces N-acyl homoserine lactones (AHLs) which apparently function as quorum-sensing signals. A Tn5 insertion mutant of DC3000, designated PsrA(-) (Psr is for Pseudomonas sigma regulator), overexpresses psyR (a LuxR-type regulator of psyI) and psyI (the gene for AHL synthase), and it produces a ca. 8-fold-higher level of AHL than does DC3000. The mutant is impaired in its ability to elicit the hypersensitive reaction and is attenuated in its virulence in tomato. These phenotypes correlate with reduced expression of hrpL, the gene for an alternate sigma factor, as well as several hrp and hop genes during early stages of incubation in a Hrp-inducing medium. PsrA also positively controls rpoS, the gene for an alternate sigma factor known to control various stress responses. By contrast, PsrA negatively regulates rsmA1, an RNA-binding protein gene known to function as negative regulator, and aefR, a tetR-like gene known to control AHL production and epiphytic fitness in P. syringae pv. syringae. Gel mobility shift assays and other lines of evidence demonstrate a direct interaction of PsrA protein with rpoS promoter DNA and aefR operator DNA. In addition, PsrA negatively autoregulates and binds the psrA operator. In an AefR(-) mutant, the expression of psyR and psyI and AHL production are lower than those in DC3000, the AefR(+) parent. In an RpoS(-) mutant, on the other hand, the levels of AHL and transcripts of psyR and psyI are much higher than those in the RpoS(+) parent, DC3000. We present evidence, albeit indirect, that the RpoS effect occurs via psyR. Thus, AefR positively regulates AHL production, whereas RpoS has a strong negative effect. We show that AefR and RpoS do not regulate PsrA and that the PsrA effect on AHL production is exerted via its cumulative, but independent, effects on both AefR and RpoS.
Figures




Similar articles
-
GacA, the response regulator of a two-component system, acts as a master regulator in Pseudomonas syringae pv. tomato DC3000 by controlling regulatory RNA, transcriptional activators, and alternate sigma factors.Mol Plant Microbe Interact. 2003 Dec;16(12):1106-17. doi: 10.1094/MPMI.2003.16.12.1106. Mol Plant Microbe Interact. 2003. PMID: 14651344
-
Characterization of each aefR and mexT mutant in Pseudomonas syringae pv. tabaci 6605.Mol Genet Genomics. 2012 Jun;287(6):473-84. doi: 10.1007/s00438-012-0693-9. Epub 2012 May 3. Mol Genet Genomics. 2012. PMID: 22552803
-
Novel virulence gene of Pseudomonas syringae pv. tomato strain DC3000.J Bacteriol. 2005 Nov;187(22):7805-14. doi: 10.1128/JB.187.22.7805-7814.2005. J Bacteriol. 2005. PMID: 16267304 Free PMC article.
-
Closing the circle on the discovery of genes encoding Hrp regulon members and type III secretion system effectors in the genomes of three model Pseudomonas syringae strains.Mol Plant Microbe Interact. 2006 Nov;19(11):1151-8. doi: 10.1094/MPMI-19-1151. Mol Plant Microbe Interact. 2006. PMID: 17073298 Review.
-
Genomic mining type III secretion system effectors in Pseudomonas syringae yields new picks for all TTSS prospectors.Trends Microbiol. 2002 Oct;10(10):462-9. doi: 10.1016/s0966-842x(02)02451-4. Trends Microbiol. 2002. PMID: 12377556 Review.
Cited by
-
Coronatine Facilitates Pseudomonas syringae Infection of Arabidopsis Leaves at Night.Front Plant Sci. 2016 Jun 21;7:880. doi: 10.3389/fpls.2016.00880. eCollection 2016. Front Plant Sci. 2016. PMID: 27446113 Free PMC article.
-
Phytopathogenic bacteria utilize host glucose as a signal to stimulate virulence through LuxR homologues.Mol Plant Pathol. 2023 Apr;24(4):359-373. doi: 10.1111/mpp.13302. Epub 2023 Feb 10. Mol Plant Pathol. 2023. PMID: 36762904 Free PMC article.
-
The Vibrio harveyi master quorum-sensing regulator, LuxR, a TetR-type protein is both an activator and a repressor: DNA recognition and binding specificity at target promoters.Mol Microbiol. 2008 Oct;70(1):76-88. doi: 10.1111/j.1365-2958.2008.06389.x. Epub 2008 Aug 4. Mol Microbiol. 2008. PMID: 18681939 Free PMC article.
-
Roles of transcriptional factor PsrA in the regulation of quorum sensing in Pseudomonas aeruginosa PAO1.Front Microbiol. 2024 Jun 26;15:1424330. doi: 10.3389/fmicb.2024.1424330. eCollection 2024. Front Microbiol. 2024. PMID: 38989021 Free PMC article.
-
Natural variation in the hrpL promoter renders the phytopathogen Pseudomonas syringae pv. actinidiae nonpathogenic.Mol Plant Pathol. 2023 Mar;24(3):262-271. doi: 10.1111/mpp.13289. Epub 2023 Jan 4. Mol Plant Pathol. 2023. PMID: 36600466 Free PMC article.
References
-
- Beattie, G. A., and S. E. Lindow. 1999. Bacterial colonization of leaves: a spectrum of strategies. Phytopathology 89:353-359. - PubMed
-
- Bertani, I., M. Sevo, M. Kojic, and V. Venturi. 2003. Role of GacA, LasI, RhlI, Ppk, PsrA, Vfr and ClpXP in the regulation of the stationary-phase sigma factor rpoS/RpoS in Pseudomonas. Arch. Microbiol. 180:264-271. - PubMed
-
- Buell, R. C., V. Joardar, M. Lindeberg, J. Selengut, I. T. Paulsen, M. L. Gwinn, R. J. Dodson, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, S. Daugherty, L. Brinkac, M. J. Beanan, D. H. Haft, W. C. Nelson, T. Davidsen, N. Zafar, L. Zhou, J. Liu, Q. Yuan, H. Khouri, N. Fedorova, B. Tran, D. Russell, K. Berry, T. Utterback, S. E. van Aken, T. V. Feldblyum, M. D'Ascenzo, W. L. Deng, A. R. Ramos, J. R. Alfano, S. Cartinhour, A. K. Chatterjee, T. P. Delaney, S. G. Lazarowitz, G. B. Martin, D. J. Schneider, X. Tang, C. L. Bender, O. White, C. M. Fraser, and A. Collmer. 2003. The complete genome sequence of the Arabidopsis and tomato pathogen Pseudomonas syringae pv. tomato DC3000. Proc. Natl. Acad. Sci. USA 100:10181-10186. - PMC - PubMed
-
- Chatterjee, A., Y. Cui, and A. K. Chatterjee. 2002. Regulation of Erwinia carotovora hrpLEcc (sigma-LEcc), which encodes an extracytoplasmic function subfamily of sigma factor required for expression of the HRP regulon. Mol. Plant-Microbe Interact. 15:971-980. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

