Identification of unconventional intestinal pathogenic Escherichia coli isolates expressing intermediate virulence factor profiles by using a novel single-step multiplex PCR
- PMID: 17400780
- PMCID: PMC1907121
- DOI: 10.1128/AEM.02855-06
Identification of unconventional intestinal pathogenic Escherichia coli isolates expressing intermediate virulence factor profiles by using a novel single-step multiplex PCR
Abstract
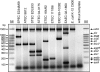
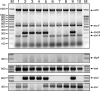
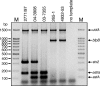
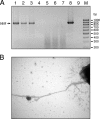
Intestinal pathogenic Escherichia coli represents a global health problem for mammals, including humans. At present, diarrheagenic E. coli bacteria are grouped into seven major pathotypes that differ in their virulence factor profiles, severity of clinical manifestations, and prognosis. In this study, we developed and evaluated a one-step multiplex PCR (MPCR) for the straightforward differential identification of intestinal pathotypes of E. coli. The specificity of this novel MPCR was validated by using a subset of reference strains and further confirmed by PCR-independent pheno- and genotypic characterization. Moreover, we tested 246 clinical E. coli isolates derived from diarrhea patients from several distinct geographic regions. Interestingly, besides strains belonging to the defined and well-described pathotypes, we identified five unconventional strains expressing intermediate virulence factor profiles. These strains have been further characterized and appear to represent intermediate strains carrying genes and expressing factors associated with enteropathogenic E. coli, Shiga toxin-producing E. coli, enterotoxigenic E. coli, and enteroaggregative E. coli alike. These strains represent further examples of the extraordinary plasticity of the E. coli genome. Moreover, this implies that the important identification of specific pathotypes has to be based on a broad matrix of indicator genes. In addition, the presence of intermediate strains needs to be accounted for.
Figures

Similar articles
-
Octaplex PCR and fluorescence-based capillary electrophoresis for identification of human diarrheagenic Escherichia coli and Shigella spp.J Microbiol Methods. 2007 Feb;68(2):331-41. doi: 10.1016/j.mimet.2006.09.013. Epub 2006 Oct 31. J Microbiol Methods. 2007. PMID: 17079041
-
Escherichia coli pathotypes associated with diarrhea in Romanian children younger than 5 years of age.Jpn J Infect Dis. 2009 Jul;62(4):289-93. Jpn J Infect Dis. 2009. PMID: 19628907
-
Characterization of Escherichia coli strains isolated from patients with diarrhea in Sao Paulo, Brazil: identification of intermediate virulence factor profiles by multiplex PCR.J Clin Microbiol. 2011 Jun;49(6):2274-8. doi: 10.1128/JCM.00386-11. Epub 2011 Apr 20. J Clin Microbiol. 2011. PMID: 21508159 Free PMC article.
-
Are Escherichia coli Pathotypes Still Relevant in the Era of Whole-Genome Sequencing?Front Cell Infect Microbiol. 2016 Nov 18;6:141. doi: 10.3389/fcimb.2016.00141. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27917373 Free PMC article. Review.
-
Updates on defining and detecting diarrheagenic Escherichia coli pathotypes.Curr Opin Infect Dis. 2020 Oct;33(5):372-380. doi: 10.1097/QCO.0000000000000665. Curr Opin Infect Dis. 2020. PMID: 32773499 Free PMC article. Review.
Cited by
-
TRS-PCR profiling for discrimination of Escherichia coli strains isolated from children with diarrhea under 5 years of age in Lodz region, Poland.Mol Biol Rep. 2016 Sep;43(9):871-80. doi: 10.1007/s11033-016-4031-x. Epub 2016 Jul 7. Mol Biol Rep. 2016. PMID: 27389591 Free PMC article.
-
DETECTION OF DIARRHEAGENIC ESCHERICHIA COLI IN HUMAN DIARRHEIC STOOL AND DRINKING WATER SAMPLES IN OUAGADOUGOU, BURKINA FASO.Afr J Infect Dis. 2020 Dec 14;15(1):53-58. doi: 10.21010/ajid.v15i1.7. eCollection 2021. Afr J Infect Dis. 2020. PMID: 33884359 Free PMC article.
-
Virulence gene profiles and molecular genetic characteristics of diarrheagenic Escherichia coli from a hospital in western China.Gut Pathog. 2018 Aug 17;10:35. doi: 10.1186/s13099-018-0262-9. eCollection 2018. Gut Pathog. 2018. PMID: 30127859 Free PMC article.
-
Resistance Patterns, mcr-4 and OXA-48 Genes, and Virulence Factors of Escherichia coli from Apennine Chamois Living in Sympatry with Domestic Species, Italy.Animals (Basel). 2022 Jan 6;12(2):129. doi: 10.3390/ani12020129. Animals (Basel). 2022. PMID: 35049753 Free PMC article.
-
Pathotyping and antimicrobial susceptibility testing of Escherichia coli isolates from neonatal calves.Vet Res Commun. 2022 Jun;46(2):353-362. doi: 10.1007/s11259-021-09857-5. Epub 2021 Nov 18. Vet Res Commun. 2022. PMID: 34796436 Free PMC article.
References
-
- Badea, L., S. Doughty, L. Nicholls, J. Sloan, R. M. Robins-Browne, and E. L. Hartland. 2003. Contribution of Efa1/LifA to the adherence of enteropathogenic Escherichia coli to epithelial cells. Microb. Pathog. 34:205-215. - PubMed
-
- Bielaszewska, M., A. W. Friedrich, T. Aldick, R. Schurk-Bulgrin, and H. Karch. 2006. Shiga toxin activatable by intestinal mucus in Escherichia coli isolated from humans: predictor for a severe clinical outcome. Clin. Infect. Dis. 43:1160-1167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

