Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans
- PMID: 17400891
- PMCID: PMC1899242
- DOI: 10.1128/EC.00399-06
Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans
Abstract
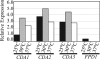
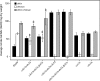
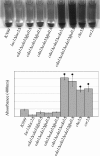
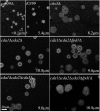
Cryptococcus neoformans is an opportunistic fungal pathogen that causes cryptococcal meningoencephalitis, particularly in immunocompromised patients. The fungal cell wall is an excellent target for antifungal therapies as it is an essential organelle that provides cell structure and integrity, it is needed for the localization or attachment of known virulence factors, including the polysaccharide capsule, melanin, and phospholipase, and it is critical for host-pathogen interactions. In C. neoformans, chitosan produced by the enzymatic removal of acetyl groups from nascent chitin polymers has been implicated as an important component of the vegetative cell wall. In this study, we identify four putative chitin/polysaccharide deacetylases in C. neoformans. We have demonstrated that three of these deacetylases, Cda1, Cda2, and Cda3, can account for all of the chitosan produced during vegetative growth in culture, but the function for one, Fpd1, remains undetermined. The data suggest a model for chitosan production in vegetatively growing C. neoformans where the three chitin deacetylases convert chitin generated by the chitin synthase Chs3 into chitosan. Utilizing a collection of chitin/polysaccharide deacetylase deletion strains, we determined that during vegetative growth, chitosan helps to maintain cell integrity and aids in bud separation. Additionally, chitosan is necessary for maintaining normal capsule width and the lack of chitosan results in a "leaky melanin" phenotype. Our analysis indicates that chitin deacetylases and the chitosan made by them may prove to be excellent antifungal targets.
Figures



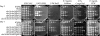
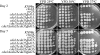
References
-
- Adams, D. J. 2004. Fungal cell wall chitinases and glucanases. Microbiology 150:2029-2035. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

