Elaboration of B gene function to include the identity of novel floral organs in the lower eudicot Aquilegia
- PMID: 17400892
- PMCID: PMC1867376
- DOI: 10.1105/tpc.107.050385
Elaboration of B gene function to include the identity of novel floral organs in the lower eudicot Aquilegia
Abstract
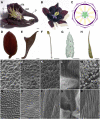
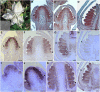
The basal eudicot Aquilegia (columbine) has an unusual floral structure that includes two morphologically distinct whorls of petaloid organs and a clearly differentiated fifth organ type, the staminodium. In this study, we have sought to determine how Aquilegia homologs of the B class genes APETALA3 (AP3) and PISTILLATA (PI) contribute to these novel forms of organ identity. Detailed expression analyses of the three AP3 paralogs and one PI homolog in wild-type and floral homeotic mutant lines reveal complex patterns that suggest that canonical B class function has been elaborated in Aquilegia. Yeast two-hybrid studies demonstrate that the protein products of Aquilegia's AP3 and PI homologs can form heterodimers, much like what has been observed for their core eudicot homologs. Downregulation of AqvPI using virus-induced gene silencing indicates that in addition to petal and stamen identity, this locus is essential to staminodial identity but may not control the identity of the petaloid sepals. Our findings show that preexisting floral organ identity programs can be partitioned and modified to produce additional organ types. In addition, they indicate that some types of petaloid organs are not entirely dependent on AP3/PI homologs for their identity.
Figures






References
-
- Aagaard, J.E., Olmstead, R.G., Willis, J.H., and Phillips, P.C. (2005). Duplication of floral regulatory genes in the Lamiales. Am. J. Bot. 92 1284–1293. - PubMed
-
- Albert, V.A., Gustafsson, M.H.G., and Di Laurenzio, L. (1998). Ontogenetic systematics, molecular developmental genetics, and the angiosperm petal. In Molecular Systematics of Plants, Vol. II, D. Soltis, P. Soltis, and J.J. Doyle, eds (New York: Chapman and Hall), pp. 349–374.
-
- Ambrose, B.A., Lerner, D.R., Ciceri, P., Padilla, C.M., Yanofsky, M.F., and Schmidt, R.J. (2000). Molecular and genetic analyses of the Silky1 gene reveal conservation in floral organ specification between eudicots and monocots. Mol. Cell 5 569–579. - PubMed
-
- Aoki, S., Uehara, K., Imafuku, M., Hasebe, M., and Ito, M. (2004). Phylogeny and divergence of basal angiosperms inferred from APETALA3- and PISTILLATA-like MADS-box genes. J. Plant Res. 117 229–244. - PubMed
-
- Becker, A., and Theissen, G. (2003). The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol. Phylogenet. Evol. 29 464–489. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

