Glucose infusion in mice: a new model to induce beta-cell replication
- PMID: 17400928
- PMCID: PMC2921922
- DOI: 10.2337/db06-1513
Glucose infusion in mice: a new model to induce beta-cell replication
Abstract
Developing new techniques to induce beta-cells to replicate is a major goal in diabetes research. Endogenous beta-cells replicate in response to metabolic changes, such as obesity and pregnancy, which increase insulin requirement. Mouse genetic models promise to reveal the pathways responsible for compensatory beta-cell replication. However, no simple, short-term, physiological replication stimulus exists to test mouse models for compensatory replication. Here, we present a new tool to induce beta-cell replication in living mice. Four-day glucose infusion is well tolerated by mice as measured by hemodynamics, body weight, organ weight, food intake, and corticosterone level. Mild sustained hyperglycemia and hyperinsulinemia induce a robust and significant fivefold increase in beta-cell replication. Glucose-induced beta-cell replication is dose and time dependent. Beta-cell mass, islet number, beta-cell size, and beta-cell death are not altered by glucose infusion over this time frame. Glucose infusion increases both the total protein abundance and nuclear localization of cyclin D2 in islets, which has not been previously reported. Thus, we have developed a new model to study the regulation of compensatory beta-cell replication, and we describe important novel characteristics of mouse beta-cell responses to glucose in the living pancreas.
Figures






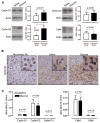
References
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. - PubMed
-
- Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88:2300–2308. - PubMed
-
- Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23:857–861. - PubMed
-
- Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic β-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38:49–53. - PubMed
-
- Lee HC, Bonner-Weir S, Weir GC, Leahy JL. Compensatory adaption to partial pancreatectomy in the rat. Endocrinology. 1989;124:1571–1575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

