Nicotine pharmacokinetics and subjective effects of three potential reduced exposure products, moist snuff and nicotine lozenge
- PMID: 17400953
- PMCID: PMC2598476
- DOI: 10.1136/tc.2006.018440
Nicotine pharmacokinetics and subjective effects of three potential reduced exposure products, moist snuff and nicotine lozenge
Abstract
Objective: To compare nicotine pharmacokinetics and subjective effects of three new smokeless tobacco potential reduced exposure products (PREPs; Ariva, Revel and Stonewall) with moist snuff (Copenhagen) and medicinal nicotine (Commit lozenge).
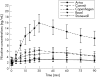
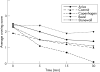
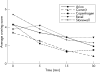
Methods: 10 subjects completed a randomised, within-subject, crossover study. Subjects used one product for 30 min at each of the five laboratory sessions. Maximal nicotine concentration (Cmax) was determined and area under the concentration time curve (AUC) was calculated for a 90-min period (during use and 60 min after use). Nicotine craving, withdrawal symptoms and ratings of product effects and liking were measured during product use.
Results: Nicotine AUC and Cmax were higher for Copenhagen than for any other product (p<0.002) and higher for Commit than for either Ariva or Revel (p<0.001). Cmax for Commit was also higher than for Stonewall (p = 0.03). Craving was lowest during use of Copenhagen (p<0.03). Craving during use of Stonewall, Ariva and Commit was lower than during use of Revel (p<0.05). Withdrawal symptom score during use of Copenhagen was lower than during use of Revel (p = 0.009). Copenhagen scores were higher (p<0.005) than all other products in several measures of drug effects and liking (feel good effects, satisfaction, liking and desire for product, and strength of product).
Conclusion: The new smokeless tobacco PREPs result in lower nicotine concentrations and equivalent or lower reductions in subjective measures compared with medicinal nicotine. Since health effects of PREPs are largely unknown, medicinal nicotine should be preferentially encouraged for smokers or smokeless tobacco users wishing to switch to lower-risk products.
Conflict of interest statement
Competing interests: None.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical