L-citrulline inhibits [3H]acetylcholine release from rat motor nerve terminals by increasing adenosine outflow and activation of A1 receptors
- PMID: 17401439
- PMCID: PMC2013966
- DOI: 10.1038/sj.bjp.0707242
L-citrulline inhibits [3H]acetylcholine release from rat motor nerve terminals by increasing adenosine outflow and activation of A1 receptors
Abstract
Background and purpose: Nitric oxide (NO) production and depression of neuromuscular transmission are closely related, but little is known about the role of L-citrulline, a co-product of NO biosynthesis, on neurotransmitter release.
Experimental approach: Muscle tension recordings and outflow experiments were performed on rat phrenic nerve-hemidiaphragm preparations stimulated electrically.
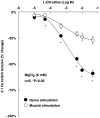
Key results: L-citrulline concentration-dependently inhibited evoked [(3)H]ACh release from motor nerve terminals and depressed nerve-evoked muscle contractions. The NO synthase (NOS) substrate, L-arginine, and the NO donor, 3-morpholinosydnonimine chloride (SIN-1), also inhibited [(3)H]ACh release with a potency order of SIN-1>L-arginine>L-citrulline. Co-application of L-citrulline and SIN-1 caused additive effects. NOS inactivation with N(omega)-nitro-L-arginine prevented L-arginine inhibition, but not that of L-citrulline. The NO scavenger, haemoglobin, abolished inhibition of [(3)H]ACh release caused by SIN-1, but not that caused by L-arginine. Inactivation of guanylyl cyclase with 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) fully blocked SIN-1 inhibition, but only partially attenuated the effects of L-arginine. Reduction of extracellular adenosine accumulation with adenosine deaminase or with the nucleoside transport inhibitor, S-(p-nitrobenzyl)-6-thioinosine, attenuated the effects of L-arginine and L-citrulline, while not affecting inhibition by SIN-1. Similar results were obtained with the selective adenosine A(1) receptor antagonist, 1,3-dipropyl-8-cyclopentylxanthine. L-citrulline increased the resting extracellular concentration of adenosine, without changing that of the adenine nucleotides.
Conclusions and implications: NOS catalyses the formation of two neuronally active products, NO and L-citrulline. While, NO may directly reduce transmitter release through stimulation of soluble guanylyl cyclase, the inhibitory action of L-citrulline may be indirect through increasing adenosine outflow and subsequently activating inhibitory A(1) receptors.
Figures

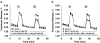

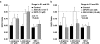

Similar articles
-
Purinergic Tuning of the Tripartite Neuromuscular Synapse.Mol Neurobiol. 2023 Jul;60(7):4084-4104. doi: 10.1007/s12035-023-03317-8. Epub 2023 Apr 5. Mol Neurobiol. 2023. PMID: 37016047 Free PMC article. Review.
-
Tuning adenosine A1 and A2A receptors activation mediates L-citrulline-induced inhibition of [3H]-acetylcholine release depending on nerve stimulation pattern.Neurochem Int. 2008 Mar-Apr;52(4-5):834-45. doi: 10.1016/j.neuint.2007.09.016. Epub 2007 Oct 3. Neurochem Int. 2008. PMID: 18022291
-
Adenosine uptake and deamination regulate tonic A2a receptor facilitation of evoked [3H]acetylcholine release from the rat motor nerve terminals.Neuroscience. 1996 Jul;73(1):85-92. doi: 10.1016/0306-4522(96)00028-0. Neuroscience. 1996. PMID: 8783232
-
Muscarinic M(3) facilitation of acetylcholine release from rat myenteric neurons depends on adenosine outflow leading to activation of excitatory A(2A) receptors.Neurogastroenterol Motil. 2009 Oct;21(10):1118-e95. doi: 10.1111/j.1365-2982.2009.01326.x. Epub 2009 May 21. Neurogastroenterol Motil. 2009. PMID: 19470085
-
Involvement of nitric oxide in neuromuscular transmission in canine proximal colon.Proc Soc Exp Biol Med. 1996 Jan;211(1):16-23. doi: 10.3181/00379727-211-43950c. Proc Soc Exp Biol Med. 1996. PMID: 8594614 Review.
Cited by
-
Novel Agonists of Adenosine Receptors in Animal Model of Acute Myocardial Infarction.Drug Des Devel Ther. 2024 Nov 15;18:5211-5223. doi: 10.2147/DDDT.S464712. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39568783 Free PMC article.
-
Purinergic Tuning of the Tripartite Neuromuscular Synapse.Mol Neurobiol. 2023 Jul;60(7):4084-4104. doi: 10.1007/s12035-023-03317-8. Epub 2023 Apr 5. Mol Neurobiol. 2023. PMID: 37016047 Free PMC article. Review.
-
Lack of endogenous adenosine tonus on sympathetic neurotransmission in spontaneously hypertensive rat mesenteric artery.PLoS One. 2014 Aug 26;9(8):e105540. doi: 10.1371/journal.pone.0105540. eCollection 2014. PLoS One. 2014. PMID: 25158061 Free PMC article.
-
Purinergic signalling in the musculoskeletal system.Purinergic Signal. 2013 Dec;9(4):541-72. doi: 10.1007/s11302-013-9381-4. Epub 2013 Aug 14. Purinergic Signal. 2013. PMID: 23943493 Free PMC article. Review.
-
Guanosine-Based Nucleotides, the Sons of a Lesser God in the Purinergic Signal Scenario of Excitable Tissues.Int J Mol Sci. 2020 Feb 26;21(5):1591. doi: 10.3390/ijms21051591. Int J Mol Sci. 2020. PMID: 32111063 Free PMC article. Review.
References
-
- Barton ER, Morris L, Kawana M, Bish LT, Toursel T. Systemic administration of L-arginine benefits mdx skeletal muscle function. Muscle Nerve. 2005;32:751–760. - PubMed
-
- Blottner D, Lück G. Just in time and place: NOS/NO system assembly in neuromuscular junction formation. Microsc Res Tech. 2001;55:171–180. - PubMed
-
- Bon CLM, Garthwaite J. Adenosine acting on A1 receptors protects NO-triggered rebound potentiation and LTP in rat hippocampal slices. J Neurophysiol. 2002;87:1781–1798. - PubMed
-
- Boulton CL, Irving AJ, Southam E, Potier B, Garthwaite J, Collingridge GL. The nitric oxide-cyclic GMP pathway and synaptic depression in rat hippocampal slices. Eur J Neurosci. 1994;6:1528–1535. - PubMed
-
- Broad RM, Fallahi N, Fredholm BB. Nitric oxide interacts with oxygen free radicals to evoke the release of adenosine and adenine nucleotides from rat hippocampal slices. J Auton Nerv Sys. 2000;81:82–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

