Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation
- PMID: 17403030
- PMCID: PMC1949347
- DOI: 10.1111/j.1471-4159.2007.04487.x
Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation
Abstract
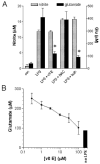
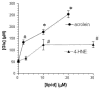
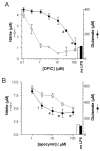
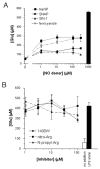
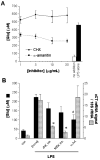
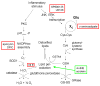
When activated by proinflammatory stimuli, microglia release substantial levels of glutamate, and mounting evidence suggests this contributes to neuronal damage during neuroinflammation. Prior studies indicated a role for the Xc exchange system, an amino acid transporter that antiports glutamate for cystine. Because cystine is used for synthesis of glutathione (GSH) synthesis, we hypothesized that glutamate release is an indirect consequence of GSH depletion by the respiratory burst, which produces superoxide from NADPH oxidase. Microglial glutamate release triggered by lipopolysaccharide was blocked by diphenylene iodonium chloride and apocynin, inhibitors of NADPH oxidase. This glutamate release was also blocked by vitamin E and elicited by lipid peroxidation products 4-hydroxynonenal and acrolein, suggesting that lipid peroxidation makes crucial demands on GSH. Although NADPH oxidase inhibitors also suppressed nitrite accumulation, vitamin E did not; moreover, glutamate release was largely unaffected by nitric oxide donors, inhibitors of nitric oxide synthase, or changes in gene expression. These findings indicate that a considerable degree of the neurodegenerative consequences of neuroinflammation may result from conversion of oxidative stress to excitotoxic stress. This phenomenon entails a biochemical chain of events initiated by a programmed oxidative stress and resultant mass-action amino acid transport. Indeed, some of the neuroprotective effects of antioxidants may be due to interference with these events rather than direct protection against neuronal oxidation.
Figures
References
-
- Alblas J, Honing H, de Lavalette CR, Brown MH, Dijkstra CD, van den Berg TK. Signal regulatory protein alpha ligation induces macrophage nitric oxide production through JAK/STAT- and phosphatidylinositol 3-kinase/Rac1/NAPDH oxidase/H2O2-dependent pathways. Mol Cell Biol. 2005;25:7181–7192. - PMC - PubMed
-
- Awasthi YC, Yang Y, Tiwari NK, Patrick B, Sharma A, Li J, Awasthi S. Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Radic Biol Med. 2004;37:607–619. - PubMed
-
- Bal-Price A, Moneer Z, Brown GC. Nitric oxide induces rapid, calcium-dependent release of vesicular glutamate and ATP from cultured rat astrocytes. Glia. 2002;40:312–323. - PubMed
-
- Barger SW. An unconventional hypothesis of oxidation in Alzheimer’s disease: intersections with excitotoxicity. Front Biosci. 2004;9:3286–3295. - PubMed
-
- Barger SW, Basile AS. Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J Neurochem. 2001;76:846–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

