A double-blind randomized study comparing the effects of continuing or not continuing rosiglitazone + metformin therapy when starting insulin therapy in people with Type 2 diabetes
- PMID: 17403121
- PMCID: PMC1974817
- DOI: 10.1111/j.1464-5491.2007.02141.x
A double-blind randomized study comparing the effects of continuing or not continuing rosiglitazone + metformin therapy when starting insulin therapy in people with Type 2 diabetes
Erratum in
- Diabet Med. 2010 May;27(5):611
Abstract
Aims: To compare the efficacy and safety of either continuing or discontinuing rosiglitazone + metformin fixed-dose combination when starting insulin therapy in people with Type 2 diabetes inadequately controlled on oral therapy.
Methods: In this 24-week double-blind study, 324 individuals with Type 2 diabetes inadequately controlled on maximum dose rosiglitazone + metformin therapy were randomly assigned to twice-daily premix insulin therapy (target pre-breakfast and pre-evening meal glucose < or = 6.5 mmol/l) in addition to either rosiglitazone + metformin (8/2000 mg) or placebo.
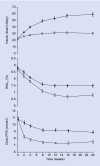
Results: Insulin dose at week 24 was significantly lower with rosiglitazone + metformin (33.5 +/- 1.5 U/day, mean +/- se) compared with placebo [59.0 +/- 3.0 U/day; model-adjusted difference -26.6 (95% CI -37.7, -15,5) U/day, P < 0.001]. Despite this, there was greater improvement in glycaemic control [HbA(1c) rosiglitazone + metformin vs. placebo 6.8 +/- 0.1 vs. 7.5 +/- 0.1%; difference -0.7 (-0.8, -0.5)%, P < 0.001] and more individuals achieved glycaemic targets (HbA(1c) < 7.0% 70 vs. 34%, P < 0.001). The proportion of individuals reporting at least one hypoglycaemic event during the last 12 weeks of treatment was similar in the two groups (rosiglitazone + metformin vs. placebo 25 vs. 27%). People receiving rosiglitazone + metformin in addition to insulin reported greater treatment satisfaction than those receiving insulin alone. Both treatment regimens were well tolerated but more participants had oedema [12 (7%) vs. 4 (3%)] and there was more weight gain [3.7 vs. 2.6 kg; difference 1.1 (0.2, 2.1) kg, P = 0.02] with rosiglitazone + metformin.
Conclusions: Addition of insulin to rosiglitazone + metformin enabled more people to reach glycaemic targets with less insulin, and was generally well tolerated.
Figures
References
-
- Klein R. The medical management of hyperglycemia over a 10-year period in people with diabetes. Diabetes Care. 1996;19:744–750. - PubMed
-
- Yki-Jarvinen H. Combination therapies with insulin in type 2 diabetes. Diabetes Care. 2001;24:758–767. - PubMed
-
- Yki-Järvinen H. Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1999;130:389–396. - PubMed
-
- Chow CC. Comparison of insulin with or without continuation of oral hypoglycemic agents in the treatment of secondary failure in NIDDM patients. Diabetes Care. 1995;18:307–314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

