Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons
- PMID: 17403372
- PMCID: PMC1913910
- DOI: 10.1016/j.cmet.2007.02.002
Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons
Abstract
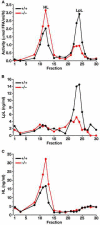
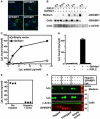
The triglycerides in chylomicrons are hydrolyzed by lipoprotein lipase (LpL) along the luminal surface of the capillaries. However, the endothelial cell molecule that facilitates chylomicron processing by LpL has not yet been defined. Here, we show that glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPIHBP1) plays a critical role in the lipolytic processing of chylomicrons. Gpihbp1-deficient mice exhibit a striking accumulation of chylomicrons in the plasma, even on a low-fat diet, resulting in milky plasma and plasma triglyceride levels as high as 5000 mg/dl. Normally, Gpihbp1 is expressed highly in heart and adipose tissue, the same tissues that express high levels of LpL. In these tissues, GPIHBP1 is located on the luminal face of the capillary endothelium. Expression of GPIHBP1 in cultured cells confers the ability to bind both LpL and chylomicrons. These studies strongly suggest that GPIHBP1 is an important platform for the LpL-mediated processing of chylomicrons in capillaries.
Figures





Comment in
-
Chylomicrons and lipoprotein lipase at the endothelial surface: bound and GAG-ged?Cell Metab. 2007 Apr;5(4):229-31. doi: 10.1016/j.cmet.2007.03.003. Cell Metab. 2007. PMID: 17403366
References
-
- Beckstead JA, Oda MN, Martin DDO, Forte TM, Bielicki JK, Berger T, Luty R, Kay CM, Ryan RO. Structure-Function Studies of Human Apolipoprotein A-V: A Regulator of Plasma Lipid Homeostasis. Biochemistry. 2003;42:9416–9423. - PubMed
-
- Bengtsson G, Olivecrona T. Apolipoprotein CII enhances hydrolysis of monoglycerides by lipoprotein lipase, but the effect is abolished by fatty acids. FEBS Lett. 1979;106:345–348. - PubMed
-
- Bensadoun A, Hsu J, Hughes B. Large-scale lipoprotein lipase purification from adipose tissue. Methods Mol. Biol. 1999;109:145–150. - PubMed
-
- Blaner WS, Obunike JC, Kurlandsky SB, al-Haideri M, Piantedosi R, Deckelbaum RJ, Goldberg IJ. Lipoprotein lipase hydrolysis of retinyl ester. Possible implications for retinoid uptake by cells. J. Biol. Chem. 1994;269:16559–16565. - PubMed
-
- Breckenridge WC, Little JA, Steiner G, Chow A, Poapst M. Hypertriglyceridemia associated with deficiency of apolipoprotein C-II. N. Engl. J. Med. 1978;298:1265–1273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

