Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver
- PMID: 17403375
- PMCID: PMC2680089
- DOI: 10.1016/j.cmet.2007.03.004
Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver
Abstract
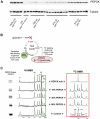
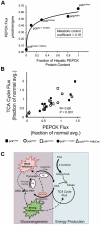
When dietary carbohydrate is unavailable, glucose required to support metabolism in vital tissues is generated via gluconeogenesis in the liver. Expression of phosphoenolpyruvate carboxykinase (PEPCK), commonly considered the control point for liver gluconeogenesis, is normally regulated by circulating hormones to match systemic glucose demand. However, this regulation fails in diabetes. Because other molecular and metabolic factors can also influence gluconeogenesis, the explicit role of PEPCK protein content in the control of gluconeogenesis was unclear. In this study, metabolic control of liver gluconeogenesis was quantified in groups of mice with varying PEPCK protein content. Surprisingly, livers with a 90% reduction in PEPCK content showed only a approximately 40% reduction in gluconeogenic flux, indicating a lower than expected capacity for PEPCK protein content to control gluconeogenesis. However, PEPCK flux correlated tightly with TCA cycle activity, suggesting that under some conditions in mice, PEPCK expression must coordinate with hepatic energy metabolism to control gluconeogenesis.
Figures
References
-
- Boden G, Chen X, Stein TP. Gluconeogenesis in mod-erately and severely hyperglycemic patients with type 2 diabetes mel-litus. Am. J. Physiol. Endocrinol. Metab. 2001;280:E23–E30. - PubMed
-
- Burgess SC, Nuss M, Chandramouli V, Hardin DS, Rice M, Landau BR, Malloy CR, Sherry AD. Analysis of gluconeogenic pathways in vivo by distribution of 2H in plasma glucose: comparison of nuclear magnetic resonance and mass spectrometry. Anal. Biochem. 2003;318:321–324. - PubMed
-
- Burgess SC, Hausler N, Merritt M, Jeffrey FMH, Storey C, Milde A, Koshy S, Lindner J, Magnuson MA, Malloy CR, Sherry AD. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J. Biol. Chem. 2004;279:48941–48949. - PubMed
-
- Burgess SC, Leone TC, Wende AR, Croce MA, Chen Z, Sherry AD, Malloy CR, Finck BN. Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha)-deficient mice. J. Biol. Chem. 2006;281:19000–19008. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

