Problems with use of composite end points in cardiovascular trials: systematic review of randomised controlled trials
- PMID: 17403713
- PMCID: PMC1852019
- DOI: 10.1136/bmj.39136.682083.AE
Problems with use of composite end points in cardiovascular trials: systematic review of randomised controlled trials
Abstract
Objective: To explore the extent to which components of composite end points in randomised controlled trials vary in importance to patients, the frequency of events in the more and less important components, and the extent of variability in the relative risk reductions across components.
Design: Systematic review of randomised controlled trials.
Data sources: Cardiovascular randomised controlled trials published in the Lancet, Annals of Internal Medicine, Circulation, European Heart Journal, JAMA, and New England Journal of Medicine, from 1 January 2002 to 30 June 2003. Component end points of composite end points were categorised according to importance to patients as fatal, critical, major, moderate, or minor.
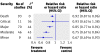
Results: Of 114 identified randomised controlled trials that included a composite end point of importance to patients, 68% (n=77) reported complete component data for the primary composite end point; almost all (98%; n=112) primary composite end points included a fatal end point. Of 84 composite end points for which component data were available, 54% (n=45) showed large or moderate gradients in both importance to patients and magnitude of effect across components. When analysed by categories of importance to patients, the most important components were associated with lower event rates in the control group (medians of 3.3-3.7% for fatal, critical, and major outcomes; 12.3% for moderate outcomes; and 8.0% for minor outcomes). Components of greater importance to patients were associated with smaller treatment effects than less important ones (relative risk reduction of 8% for death and 33% for components of minor importance to patients).
Conclusion: The use of composite end points in cardiovascular trials is frequently complicated by large gradients in importance to patients and in magnitude of the effect of treatment across component end points. Higher event rates and larger treatment effects associated with less important components may result in misleading impressions of the impact of treatment.
Conflict of interest statement
Figures


Comment in
-
Composite and surrogate outcomes in randomised controlled trials.BMJ. 2007 Apr 14;334(7597):756-7. doi: 10.1136/bmj.39176.461227.80. BMJ. 2007. PMID: 17431231 Free PMC article.
References
-
- Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials: greater precision but with greater uncertainty? JAMA 2003;289:2554-9. - PubMed
-
- Ferreira-González I, Permanyer-Miralda G, Busse JW, Bryant DM, Montori VM, Alonso-Coello P, et al. Rationale for using primary composite endpoints in clinical trials: a systematic review. J Clin Epidemiol (in press).
-
- Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6. Updated September 2006. .www.cochrane.org/resources/handbook/Handbook4.2.6Sep2006.doc
-
- Guyatt G, Montori V, Devereaux PJ, Schunemann H, Bhandari M. Patients at the center: in our practice, and in our use of language. ACP J Club 2004;140:A11-2. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
