U2 toggles iteratively between the stem IIa and stem IIc conformations to promote pre-mRNA splicing
- PMID: 17403782
- PMCID: PMC1838533
- DOI: 10.1101/gad.1536107
U2 toggles iteratively between the stem IIa and stem IIc conformations to promote pre-mRNA splicing
Abstract
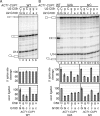
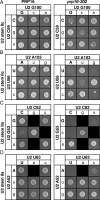
To ligate exons in pre-messenger RNA (pre-mRNA) splicing, the spliceosome must reposition the substrate after cleaving the 5' splice site. Because spliceosomal small nuclear RNAs (snRNAs) bind the substrate, snRNA structures may rearrange to reposition the substrate. However, such rearrangements have remained undefined. Although U2 stem IIc inhibits binding of U2 snRNP to pre-mRNA during assembly, we found that weakening U2 stem IIc suppressed a mutation in prp16, a DExD/H box ATPase that promotes splicing after 5' splice site cleavage. The prp16 mutation was also suppressed by mutations flanking stem IIc, suggesting that Prp16p facilitates a switch from stem IIc to the mutually exclusive U2 stem IIa, which activates binding of U2 to pre-mRNA during assembly. Providing evidence that stem IIa switches back to stem IIc before exon ligation, disrupting stem IIa suppressed 3' splice site mutations, and disrupting stem IIc impaired exon ligation. Disrupting stem IIc also exacerbated the 5' splice site cleavage defects of certain substrate mutations, suggesting a parallel role for stem IIc at both catalytic stages. We propose that U2, much like the ribosome, toggles between two conformations--a closed stem IIc conformation that promotes catalysis and an open stem IIa conformation that promotes substrate binding and release.
Figures


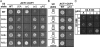


References
-
- Ares M., Jr., Igel A.H., Igel A.H. Lethal and temperature-sensitive mutations and their suppressors identify an essential structural element in U2 small nuclear RNA. Genes & Dev. 1990;12A:2132–2145. - PubMed
-
- Brow D.A., Guthrie C., Guthrie C. Spliceosomal RNA U6 is remarkably conserved from yeast to mammals. Nature. 1988;6179:213–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
