Moraxella catarrhalis outer membrane protein CD elicits antibodies that inhibit CD binding to human mucin and enhance pulmonary clearance of M. catarrhalis in a mouse model
- PMID: 17403868
- PMCID: PMC1932855
- DOI: 10.1128/IAI.00074-07
Moraxella catarrhalis outer membrane protein CD elicits antibodies that inhibit CD binding to human mucin and enhance pulmonary clearance of M. catarrhalis in a mouse model
Abstract
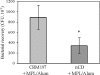
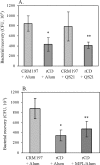
The outer membrane protein CD of Moraxella catarrhalis is considered to be a potential vaccine antigen against Moraxella infection. We purified the native CD from isolate O35E, administered it to mice, and detected considerable titers of anti-CD antibodies. Anti-CD sera were cross-reactive towards six different M. catarrhalis isolates and promoted bacterial clearance of O35E in a pulmonary challenge model. To circumvent the difficulty of generating large quantities of CD from M. catarrhalis for vaccine use, the CD gene from O35E was cloned into Escherichia coli, and the recombinant CD, expressed without a signal sequence or fusion tags, represented approximately 70% of the total E. coli proteins. The recombinant CD formed inclusion bodies that were solubilized with 6 M urea and then purified by ion-exchange chromatography, a procedure that produced soluble CD of high purity and yield. Mice immunized with the purified recombinant CD had significant titers of anti-CD antibodies that were cross-reactive towards 24 different M. catarrhalis isolates. Upon challenge, these mice showed enhanced bacterial clearance of both O35E and a heterologous M. catarrhalis isolate, TTA24. In an in vitro assay, antisera to either the native or the recombinant CD inhibited the binding activity of CD to human tracheobronchial mucin in a serum concentration-dependent manner, and the extent of inhibition appeared to correlate with the corresponding anti-CD antibody titer and whole-cell enzyme-linked immunosorbent assay titer. Our results demonstrate that the recombinant CD is a promising vaccine candidate for preventing Moraxella infection.
Figures

References
-
- Barreiro, B., L. Estaban, E. Prats, E. Verdaguer, J. Dorca, and F. Manresa. 1992. Branhamella catarrhalis respiratory infections. Eur. Respir. J. 5:675-679. - PubMed
-
- Berk, S. L., and J. H. Kalbfleisch. 1996. Antibiotic susceptibility patterns of community-acquired respiratory isolates of Moraxella catarrhalis in Western Europe and in the USA. J. Antimicrob. Chemother. 38:85-96. - PubMed
-
- Berner, R., R. F. Schumacher, M. Brandis, and J. Forster. 1996. Colonization and infection with Moraxella catarrhalis in childhood. Eur. J. Clin. Microbiol. Infect. Dis. 15:506-509. - PubMed
-
- Bernstein, J. M., and M. Reddy. 2000. Bacteria-mucin interaction in the upper aerodigestive tract shows striking heterogeneity: implications in otitis media, rhinosinusitis, and pneumonia. Otolaryngol. Head Neck Surg. 122:514-520. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

