Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection
- PMID: 17403873
- PMCID: PMC1932856
- DOI: 10.1128/IAI.01329-06
Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection
Abstract
Host defense mechanisms against Pneumocystis carinii are not fully understood. Previous work in the murine model has shown that host defense against infection is critically dependent upon host CD4(+) T cells. The recently described Th17 immune response is predominantly a function of effector CD4(+) T cells stimulated by interleukin-23 (IL-23), but whether these cells are required for defense against P. carinii infection is unknown. We tested the hypothesis that P. carinii stimulates the early release of IL-23, leading to increases in IL-17 production and lung effector CD4(+) T-cell population that mediate clearance of infection. In vitro, stimulation of alveolar macrophages with P. carinii induced IL-23, and IL-23p19 mRNA was expressed in lungs of mice infected with this pathogen. To address the role of IL-23 in resistance to P. carinii, IL-23p19-/- and wild-type control C57BL/6 mice were infected and their fungal burdens and cytokine/chemokine responses were compared. IL-23p19-/- mice displayed transient but impaired clearance of infection, which was most apparent 2 weeks after inoculation. In confirmatory studies, the administration of either anti-IL-23p19 or anti-IL-17 neutralizing antibody to wild-type mice infected with P. carinii also caused increases in fungal burdens. IL-17 and the lymphocyte chemokines IP-10, MIG, MIP-1alpha, MIP-1beta, and RANTES were decreased in the lungs of infected IL-23p19-/- mice in comparison to their levels in the lungs of wild-type mice. In IL-23p19-/- mice infected with P. carinii, there were fewer effector CD4(+) T cells in the lung tissue. Collectively, these studies indicate that the IL-23-IL-17 axis participates in host defense against P. carinii.
Figures
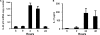
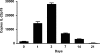
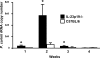

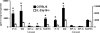
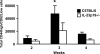
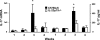
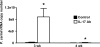
References
-
- Aggarwal, S., N. Ghilardi, M. H. Xie, F. J. de Sauvage, and A. L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910-1914. - PubMed
-
- Bang, D., J. Emborg, J. Elkjaer, J. D. Lundgren, and T. L. Benfield. 2001. Independent risk of mechanical ventilation for AIDS-related Pneumocystis carinii pneumonia associated with bronchoalveolar lavage neutrophilia. Respir. Med. 95:661-665. - PubMed
-
- Beck, J., M. Warnock, J. Curtis, M. Sniezek, S. Arrag-Peffer, H. Kaltreider, and J. Shellito. 1991. Inflammatory responses to Pneumocystis carinii in mice selectively depleted of helper T lymphocytes. Am. J. Respir. Cell Mol. Biol. 5:186-197. - PubMed
-
- Carr, J. A., J. A. Rogerson, M. J. Mulqueen, N. A. Roberts, and A. A. Nash. 1999. The role of endogenous interleukin-12 in resistance to murine cytomegalovirus (MCMV) infection and a novel action for endogenous IL-12 p40. J. Interferon Cytokine Res. 19:1145-1152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

