Combinatorial expression of alpha- and gamma-protocadherins alters their presenilin-dependent processing
- PMID: 17403907
- PMCID: PMC1900011
- DOI: 10.1128/MCB.01708-06
Combinatorial expression of alpha- and gamma-protocadherins alters their presenilin-dependent processing
Abstract
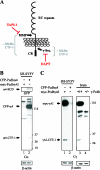
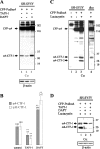
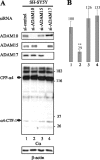
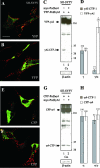
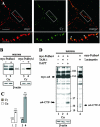
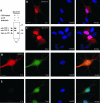
Alpha- and gamma-protocadherins (Pcdhs) are type I transmembrane receptors expressed predominantly in the central nervous system and located in part in synapses. They are transcribed from complex genomic loci, giving rise in the mouse to 14 alpha-Pcdh and 22 gamma-Pcdh isoforms consisting of variable domains, each encompassing the extracellular region, the transmembrane region, and part of the intracellular region harboring the alpha- or gamma-Pcdh-specific invariant cytoplasmic domain. Presenilin-dependent intramembrane proteolysis (PS-IP) of gamma-Pcdhs and the formation of alpha/gamma-Pcdh heteromers led us to investigate the effects of homo- and heteromer formation on gamma- and putative alpha-Pcdh membrane processing and signaling. We find that upon surface delivery, alpha-Pcdhs, like gamma-Pcdhs, are subject to matrix metallo-protease cleavage followed by PS-IP in neurons. We further demonstrate that the combinatorial expression of alpha- and gamma-Pcdhs modulates the extent of their PS-IP, indicating the formation of alpha/gamma-Pcdh heteromers with an altered susceptibility to processing. Cell-specific expression of alpha/gamma-Pcdh isoforms could thus determine cell and synapse adhesive properties as well as intracellular and nuclear signaling by their soluble cytoplasmic cleavage products, alpha C-terminal fragment 2 (alpha-CTF-2) and gamma-CTF-2.
Figures



References
-
- Brown, M. S., and J. L. Goldstein. 1997. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89:331-340. - PubMed
-
- Dittgen, T., A. Nimmerjahn, S. Komai, P. Licznerski, J. Waters, T. W. Margrie, F. Helmchen, W. Denk, M. Brecht, and P. Osten. 2004. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc. Natl. Acad. Sci. USA 101:18206-18211. - PMC - PubMed
-
- Esumi, S., N. Kakazu, Y. Taguchi, T. Hirayama, A. Sasaki, T. Hirabayashi, T. Koide, T. Kitsukawa, S. Hamada, and T. Yagi. 2005. Monoallelic yet combinatorial expression of variable exons of the protocadherin-alpha gene cluster in single neurons. Nat. Genet. 37:171-176. - PubMed
-
- Fenteany, G., R. F. Standaert, W. S. Lane, S. Choi, E. J. Corey, and S. L. Schreiber. 1995. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science 268:726-731. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
