Spontaneous tumor rejection by cbl-b-deficient CD8+ T cells
- PMID: 17403934
- PMCID: PMC2118550
- DOI: 10.1084/jem.20061699
Spontaneous tumor rejection by cbl-b-deficient CD8+ T cells
Abstract
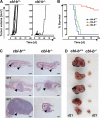
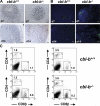
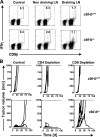
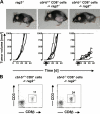
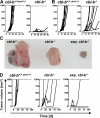
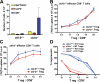
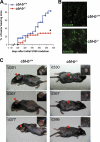
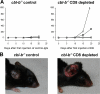
The concept of tumor surveillance implies that specific and nonspecific components of the immune system eliminate tumors in the early phase of malignancy. Understanding the biochemical mechanisms of tumor immunosurveillance is of paramount significance because it might allow one to specifically modulate spontaneous antitumor activity. We report that inactivation of the E3 ligase Casitas B cell lymphoma-b (Cbl-b) confers spontaneous in vivo rejection of tumor cells that express human papilloma virus antigens. Moreover, cbl-b(-/-) mice develop significantly fewer ultraviolet B (UVB)-induced skin malignancies and reject UVB-induced skin tumors. CD8(+) T cells were identified as key players in the spontaneous tumor rejection response. Loss of Cbl-b not only enhances antitumor reactivity of CD8(+) T cells but also occurs in the absence of CD4(+) T cells. Mechanistically, cbl-b(-/-) CD8(+) T cells are resistant to T regulatory cell-mediated suppression and exhibit enhanced activation and rapid tumor infiltration. Importantly, therapeutic transfer of naive cbl-b(-/-) CD8(+) T cells is sufficient to mediate rejection of established tumors. Even up to 1 yr after the first encounter with the tumor cells, cbl-b(-/-) mice carry an "anticancer memory." These data identify Cbl-b as a key signaling molecule that controls spontaneous antitumor activity of cytotoxic T cells in different cancer models. Inhibition of Cbl-b is a novel approach to stimulate long-lasting immunity against cancer.
Figures
References
-
- Pardoll, D.M. 1993. New strategies for enhancing the immunogenicity of tumors. Curr. Opin. Immunol. 5:719–725. - PubMed
-
- Burnet, F.M. 1970. The concept of immunological surveillance. Prog. Exp. Tumor Res. 13:1–27. - PubMed
-
- Dunn, G.P., L.J. Old, and R.D. Schreiber. 2004. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 21:137–148. - PubMed
-
- Speiser, D.E., R. Miranda, A. Zakarian, M.F. Bachmann, K. McKall-Faienza, B. Odermatt, D. Hanahan, R.M. Zinkernagel, and P.S. Ohashi. 1997. Self antigens expressed by solid tumors do not efficiently stimulate naive or activated T cells: implications for immunotherapy. J. Exp. Med. 186:645–653. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

