Magnetically driven plasmid DNA delivery with biodegradable polymeric nanoparticles
- PMID: 17403937
- PMCID: PMC3378388
- DOI: 10.1096/fj.06-8070com
Magnetically driven plasmid DNA delivery with biodegradable polymeric nanoparticles
Abstract
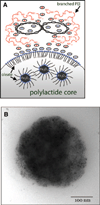
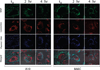
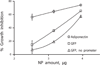
Targeting gene therapy remains a challenge. The use of magnetic force to achieve this was investigated in the present study. It was hypothesized that nanoparticles with both controllable particle size and magnetic properties would enable magnetically driven gene delivery. We investigated this hypothesis by creating a family of novel biodegradable polymeric superparamagnetic nanoparticle (MNP) formulations. Polylactide MNP were formulated using a modified emulsification-solvent evaporation methodology with both the incorporation of oleate-coated iron oxide and a polyethylenimine (PEI) oleate ion-pair surface modification for DNA binding. MNP size could be controlled by varying the proportion of the tetrahydrofuran cosolvent. Magnetically driven MNP-mediated gene transfer was studied using a green fluorescent protein reporter plasmid in cultured arterial smooth muscle cells and endothelial cells. MNP-DNA internalization and trafficking were examined by confocal microscopy. Cell growth inhibition after MNP-mediated adiponectin plasmid transfection was studied as an example of a therapeutic end point. MNP-DNA complexes protected DNA from degradation and efficiently transfected quiescent cells under both low and high serum conditions after a 15 min exposure to a magnetic field (500 G). There was negligible transfection with MNP in the absence of a magnetic field. Larger sized MNP (375 nm diameter) exhibited higher transfection rates compared with 185 nm- and 240 nm-sized MNP. Internalized larger sized MNP escaped lysosomal localization and released DNA in the perinuclear zone. Adiponectin plasmid DNA delivery using MNP resulted in a dose-dependent growth inhibition of cultured arterial smooth muscle cells. It is concluded that magnetically driven plasmid DNA delivery can be achieved using biodegradable MNP containing oleate-coated magnetite and surface modified with PEI oleate ion-pair complexes that enable DNA binding.
Figures

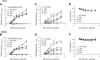
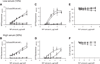
References
-
- Barry ME, Pinto-Gonzalez D, Orson FM, McKenzie GJ, Petry GR, Barry MA. Role of endogenous endonucleases and tissue site in transfection and CpG-mediated immune activation after naked DNA injection. Hum. Gene Ther. 1999;10:2461–2480. - PubMed
-
- Lechardeur D, Sohn KJ, Haardt M, Joshi PB, Monck M, Graham RW, Beatty B, Squire J, O’Brodovich H, Lukacs GL. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene Ther. 1999;6:482–497. - PubMed
-
- Rolland A. Nuclear gene delivery: the Trojan horse approach. Expert Opin. Drug Deliv. 2006;3:1–10. - PubMed
-
- Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Krüger A, Gansbacher B, Plank C. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9:102–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

