Lipoprotein SmpA is a component of the YaeT complex that assembles outer membrane proteins in Escherichia coli
- PMID: 17404237
- PMCID: PMC1851043
- DOI: 10.1073/pnas.0701579104
Lipoprotein SmpA is a component of the YaeT complex that assembles outer membrane proteins in Escherichia coli
Abstract
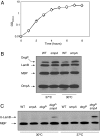
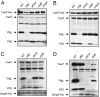
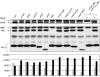
A major role of the outer membrane (OM) of Gram-negative bacteria is to provide a protective permeability barrier for the cell, and proper maintenance of the OM is required for cellular viability. OM biogenesis requires the coordinated assembly of constituent lipids and proteins via dedicated OM assembly machineries. We have previously shown that, in Escherichia coli, the multicomponent YaeT complex is responsible for the assembly of OM beta-barrel proteins (OMPs). This complex contains the OMP YaeT and three OM lipoproteins. Here, we report another component of the YaeT complex, the OM lipoprotein small protein A (SmpA). Strains carrying loss-of-function mutations in smpA are viable but exhibit defects in OMP assembly. Biochemical experiments show that SmpA is involved in maintaining complex stability. Taken together, these experiments establish an important role for SmpA in both the structure and function of the YaeT complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

