Wnt signaling regulates pancreatic beta cell proliferation
- PMID: 17404238
- PMCID: PMC1847455
- DOI: 10.1073/pnas.0701509104
Wnt signaling regulates pancreatic beta cell proliferation
Abstract
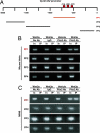
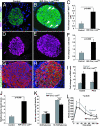
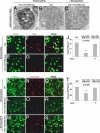
There is widespread interest in defining factors and mechanisms that stimulate proliferation of pancreatic islet cells. Wnt signaling is an important regulator of organ growth and cell fates, and genes encoding Wnt-signaling factors are expressed in the pancreas. However, it is unclear whether Wnt signaling regulates pancreatic islet proliferation and differentiation. Here we provide evidence that Wnt signaling stimulates islet beta cell proliferation. The addition of purified Wnt3a protein to cultured beta cells or islets promoted expression of Pitx2, a direct target of Wnt signaling, and Cyclin D2, an essential regulator of beta cell cycle progression, and led to increased beta cell proliferation in vitro. Conditional pancreatic beta cell expression of activated beta-catenin, a crucial Wnt signal transduction protein, produced similar phenotypes in vivo, leading to beta cell expansion, increased insulin production and serum levels, and enhanced glucose handling. Conditional beta cell expression of Axin, a potent negative regulator of Wnt signaling, led to reduced Pitx2 and Cyclin D2 expression by beta cells, resulting in reduced neonatal beta cell expansion and mass and impaired glucose tolerance. Thus, Wnt signaling is both necessary and sufficient for islet beta cell proliferation, and our study provides previously unrecognized evidence of a mechanism governing endocrine pancreas growth and function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
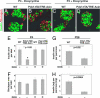
References
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Diabetes. 2003;52:102–110. - PubMed
-
- Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Diabetes. 2005;54:2060–2069. - PubMed
-
- Heit JJ, Karnik SK, Kim SK. Annu Rev Cell Dev Biol. 2006;22:311–338. - PubMed
-
- Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocana A, Vasavada R, Stewart AF. Endocr Rev. 2006;27:356–370. - PubMed
-
- Logan CY, Nusse R. Annu Rev Cell Dev Biol. 2004;20:781–810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

