Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation
- PMID: 17405864
- PMCID: PMC1851071
- DOI: 10.1073/pnas.0608647104
Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation
Abstract
Upon vascular injury, locally controlled haemostasis prevents life-threatening blood loss and ensures wound healing. Intracellular material derived from damaged cells at these sites will become exposed to blood components and could contribute to blood coagulation and pathological thrombus formation. So far, the functional and mechanistic consequences of this concept are not understood. Here, we present in vivo and in vitro evidence that different forms of eukaryotic and prokaryotic RNA serve as promoters of blood coagulation. Extracellular RNA was found to augment (auto-)activation of proteases of the contact phase pathway of blood coagulation such as factors XII and XI, both exhibiting strong RNA binding. Moreover, administration of exogenous RNA provoked a significant procoagulant response in rabbits. In mice that underwent an arterial thrombosis model, extracellular RNA was found associated with fibrin-rich thrombi, and pretreatment with RNase (but not DNase) significantly delayed occlusive thrombus formation. Thus, extracellular RNA derived from damaged or necrotic cells particularly under pathological conditions or severe tissue damage represents the long sought natural "foreign surface" and provides a procoagulant cofactor template for the factors XII/XI-induced contact activation/amplification of blood coagulation. Extracellular RNA thereby reveals a yet unrecognized target for antithrombotic intervention, using RNase or related therapeutic strategies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
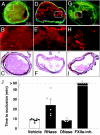


References
-
- Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmermann GA, McEver RP, Pober JS, Wicj TM, Konkle BA, Schwartz BS, et al. Blood. 1998;91:3527–3561. - PubMed
-
- Ruf W, Mueller BM. Thromb Haemost. 1999;82:175–182. - PubMed
-
- Mann KG, Butenas S, Brummel K. Arterioscler Thromb Vasc Biol. 2003;23:17–25. - PubMed
-
- Müller I, Klocke A, Alex M, Kotzsch M, Luther T, Morgenstern E, Zieseniss S, Zahler S, Preissner KT, Engelmann B. FASEB J. 2003;17:476–478. - PubMed
-
- Davie EW, Ratnoff OD. Science. 1964;145:1310–1312. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

