Production of chimeras by aggregation of embryonic stem cells with diploid or tetraploid mouse embryos
- PMID: 17406396
- PMCID: PMC2883166
- DOI: 10.1038/nprot.2006.173
Production of chimeras by aggregation of embryonic stem cells with diploid or tetraploid mouse embryos
Abstract
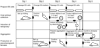
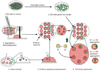
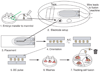
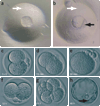
The production of mouse chimeras is a common step in the establishment of genetically modified animal strains. Chimeras also provide a powerful experimental tool for following cell behavior during both prenatal and postnatal development. This protocol outlines a simple and economical technique for the production of large numbers of mouse chimeras using traditional diploid morula<-->diploid embryonic stem (ES) cell aggregations. Additional steps are included to describe the procedures necessary to produce specialized tetraploid chimeras using tetraploid morula<-->diploid ES cell aggregations. This increasingly popular form of chimera produces embryos of nearly complete ES cell derivation that can be used to speed transgenic production or ask developmental questions. Using this protocol, mouse chimeras can be generated and transferred to pseudopregnant surrogate mothers in a 5-d period.
Figures
References
-
- Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. - PubMed
-
- Thompson S, Clarke AR, Pow AM, Hooper ML, Melton DW. Germ line transmission and expression of a corrected HPRT gene produced by gene targeting in embryonic stem cells. Cell. 1989;56:313–321. - PubMed
-
- Tam PP, Rossant J. Mouse embryonic chimeras: tools for studying mammalian development. Development. 2003;130:6155–6163. - PubMed
-
- Kunath T, et al. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development. 2005;132:1649–1661. - PubMed
-
- Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

