Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice
- PMID: 17406675
- PMCID: PMC1831495
- DOI: 10.1371/journal.pone.0000351
Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice
Abstract
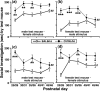
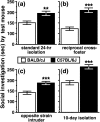
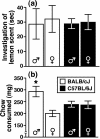
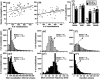
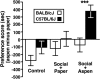
Social approach is crucial for establishing relationships among individuals. In rodents, social approach has been studied primarily within the context of behavioral phenomena related to sexual reproduction, such as mating, territory defense and parental care. However, many forms of social interaction occur before the onset of reproductive maturity, which suggests that some processes underlying social approach among juvenile animals are probably distinct from those in adults. We conducted a longitudinal study of social investigation (SI) in mice from two inbred strains to assess the extent to which genetic factors influence the motivation for young mice to approach one another. Early-adolescent C57BL/6J (B6) mice, tested 4-6 days after weaning, investigated former cage mates to a greater degree than BALB/cJ (BALB) mice, irrespective of the sex composition within an interacting pair. This strain difference was not due to variation in maternal care, the phenotypic characteristics of stimulus mice or sensitivity to the length of isolation prior to testing, nor was it attributable to a general difference in appetitive motivation. Ultrasonic vocalization (USV) production was positively correlated with the SI responses of mice from both strains. Interestingly, several USV characteristics segregated with the genetic background of young mice, including a higher average frequency and shorter duration for the USVs emitted by B6 mice. An assessment of conditioned place preference responses indicated that there was a strain-dependent difference in the rewarding nature of social contact. As adolescent mice aged, SI responses gradually became less sensitive to genetic background and more responsive to the particular sex of individuals within an interacting pair. We have thus identified a specific, genetic influence on the motivation of early-adolescent mice to approach one another. Consistent with classical theories of motivation, which propose a functional relationship between behavioral approach and reward, our findings indicate that reward is a proximal mechanism through which genetic factors affect social motivation during early adolescence.
Conflict of interest statement
Figures



References
-
- Moles A, D'amato FR. Ultrasonic vocalization by female mice in the presence of a conspecific carrying food cues. Anim Behav. 2000;60:689–694. - PubMed
-
- Siviy SM, Panksepp J. Energy balance and play in juvenile rats. Physiol Behav. 1985;35:435–441. - PubMed
-
- Barash DP. The evolution of marmot societies: A general theory. Science. 1974;185:415–420. - PubMed
-
- Freeland WJ. Parasitism and behavioral dominance among male mice. Science. 1981;213:461–462. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

