Prolonged expression of a lysosomal enzyme in mouse liver after Sleeping Beauty transposon-mediated gene delivery: implications for non-viral gene therapy of mucopolysaccharidoses
- PMID: 17407189
- PMCID: PMC1868578
- DOI: 10.1002/jgm.1028
Prolonged expression of a lysosomal enzyme in mouse liver after Sleeping Beauty transposon-mediated gene delivery: implications for non-viral gene therapy of mucopolysaccharidoses
Abstract
Background: The Sleeping Beauty (SB) transposon system is a non-viral vector system that can integrate precise sequences into chromosomes. We evaluated the SB transposon system as a tool for gene therapy of mucopolysaccharidosis (MPS) types I and VII.
Methods: We constructed SB transposon plasmids for high-level expression of human beta-glucuronidase (hGUSB) or alpha-L-iduronidase (hIDUA). Plasmids were delivered with and without SB transposase to mouse liver by rapid, high-volume tail-vein injection. We studied the duration of expressed therapeutic enzyme activity, transgene presence by PCR, lysosomal pathology by toluidine blue staining and cell-mediated immune response histologically and by immunohistochemical staining.
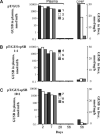
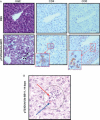
Results: Transgene frequency, distribution of transgene and enzyme expression in liver and the level of transgenic enzyme required for amelioration of lysosomal pathology were estimated in MPS I and VII mice. Without immunomodulation, initial GUSB and IDUA activities in plasma reached > 100-fold of wild-type (WT) levels but fell to background within 4 weeks post-injection. In immunomodulated transposon-treated MPS I mice plasma IDUA persisted for over 3 months at up to 100-fold WT activity in one-third of MPS I mice, which was sufficient to reverse lysosomal pathology in the liver and, partially, in distant organs. Histological and immunohistochemical examination of liver sections in IDUA transposon-treated WT mice revealed inflammation 10 days post-injection consisting predominantly of mononuclear cells, some of which were CD4- or CD8-positive.
Conclusions: Our results demonstrate the feasibility of achieving prolonged expression of lysosomal enzymes in the liver and reversing MPS disease in adult mice with a single dose of therapeutic SB transposons.
Copyright (c) 2007 John Wiley & Sons, Ltd.
Figures





References
-
- Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, et al., editors. The Metabolic and Molecular Bases of Inherited Disease. 8th edn III. McGraw-Hill; New York: 2001. pp. 3427–3436.
-
- Sly WS. Enzyme replacement therapy: from concept to clinical practice. Acta Paediatr Suppl. 2002;91:71–78. - PubMed
-
- Wraith JE. Limitations of enzyme replacement therapy: current and future. J Inherit Metab Dis. 2006;29:442–447. - PubMed
-
- Whitley CB, Belani KG, Chang PN, et al. Long-term outcome of Hurler syndrome following bone marrow transplantation. Am J Med Genet. 1993;46:209–218. - PubMed
-
- Krivit W, Aubourg P, Shapiro E, Peters C. Bone marrow transplantation for globoid cell leukodystrophy, adrenoleukodystrophy, metachromatic leukodystrophy, and Hurler syndrome. Curr Opin Hematol. 1999;6:377–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

