The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention
- PMID: 17407548
- PMCID: PMC1851974
- DOI: 10.1186/1476-4598-6-24
The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention
Abstract
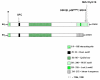
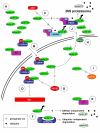
Cyclin D1 is an important regulator of cell cycle progression and can function as a transcriptionl co-regulator. The overexpression of cyclin D1 has been linked to the development and progression of cancer. Deregulated cyclin D1 degradation appears to be responsible for the increased levels of cyclin D1 in several cancers. Recent findings have identified novel mechanisms involved in the regulation of cyclin D1 stability. A number of therapeutic agents have been shown to induce cyclin D1 degradation. The therapeutic ablation of cyclin D1 may be useful for the prevention and treatment of cancer. In this review, current knowledge on the regulation of cyclin D1 degradation is discussed. Novel insights into cyclin D1 degradation are also discussed in the context of ablative therapy. A number of unresolved questions regarding the regulation of cellular cyclin D1 levels are also addressed.
Figures
References
-
- Kato J, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

