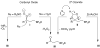Ozonolysis of unsaturated organotrifluoroborates
- PMID: 17408287
- PMCID: PMC2504467
- DOI: 10.1021/jo070130r
Ozonolysis of unsaturated organotrifluoroborates
Abstract
Organotrifluoroborates are robust reagents capable of withstanding ozonolysis of remote alkenes, thus providing a new route to oxo-substituted organotrifluoroborates. The primary ozonides initially generated upon ozonolysis can be reduced with Zn/AcOH to afford the carbonyl compounds. Alternatively, capture of the carbonyl oxides with either an appropriate N-oxide or H2O easily gives the desired oxo-substituted organotrifluoroborates. Both unsaturated alkyltrifluoroborates and aryltrifluoroborates effectively participate in the reaction. The process provides oxo-functionalized organotrifluoroborates that cannot be prepared directly via either transmetalation or hydroboration protocols.
Figures
References
-
- Darses S, Michaud G, Genet JP. Tetrahedron Lett. 1998;39:5045–5048.
- Darses S, Genet JP, Brayer JL, Demoute JP. Tetrahedron Lett. 1997;38:4393–4396.
- Xia M, Chen Z-C. Synth Commun. 1999;29:2457–2465.
- Batey RA, Quach TD. Tetrahedron Lett. 2001;42:9099–9103.
-
- Batey RA, Thadani AN, Smil DV. Org Lett. 1999;1:1683–1686.
-
- Pucheault M, Darses S, Genet JP. Eur J Org Chem. 2002:3552–3557.
-
- Thadani AN, Batey RA. Org Lett. 2002;4:3827–3830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical