Dynamics of in silico leukocyte rolling, activation, and adhesion
- PMID: 17408504
- PMCID: PMC1839892
- DOI: 10.1186/1752-0509-1-14
Dynamics of in silico leukocyte rolling, activation, and adhesion
Abstract
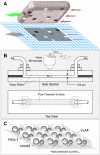
Background: We present a multilevel, agent based, in silico model that represents the dynamics of rolling, activation, and adhesion of individual leukocytes in vitro. Object-oriented software components were designed, verified, plugged together, and then operated in ways that represent the molecular and cellular mechanisms believed responsible for leukocyte rolling and adhesion. The result is an in silico analogue of an experimental in vitro system. The experimentally measured, phenotypic attributes of the analogue were compared and contrasted to those of leukocytes in vitro from three different experimental conditions.
Results: The individual in silico dynamics of "rolling" on simulated P-selectin, and separately on simulated VCAM-1, were an acceptable match to individual in vitro distance-time and velocity-time measurements. The analogues are also able to represent the transition from rolling to adhesion on P-selectin and VCAM-1 in the presence of GRO-alpha chemokine. The individual in silico and in vitro behavioral similarities translated successfully to population level measures. These behavioral similarities were enabled in part by subdividing the functionality of the analogue's surface into 600 independent, "cell"-controlled, equally capable modules of comparable functionality.
Conclusion: The overlap in phenotypic attributes of our analogue with those of leukocytes in vitro confirm the considerable potential of our model for studying the key events that determine the behavioral outcome of individual leukocytes during rolling, activation, and adhesion. Our results provide an important foundation and framework for future in silico research into plausible causal links between well-documented, subcellular molecular level events and the variety of systemic phenotypic attributes that distinguish normal leukocyte adhesion from abnormal disease-associated adhesion.
Figures










References
-
- Hamann A, Engelhardt B. Leukocyte trafficking: molecular mechanisms, therapeutic targets, and methods. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2005.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

