Exploration of prostanoid receptor subtype regulating estradiol and prostaglandin E2 induction of spinophilin in developing preoptic area neurons
- PMID: 17408863
- PMCID: PMC1945818
- DOI: 10.1016/j.neuroscience.2007.02.006
Exploration of prostanoid receptor subtype regulating estradiol and prostaglandin E2 induction of spinophilin in developing preoptic area neurons
Abstract
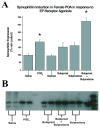
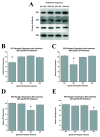
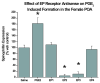
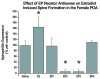
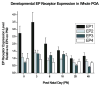
The prostaglandin E2 (PGE2) mediates estradiol-induced masculinization of sexual behavior in the rat during a perinatal sensitive period. PGE2 induces formation of dendritic spines on preoptic area (POA) neurons and this synaptic pattern change is associated with the ability to express male sexual behavior as an adult. Whether PGE2 is released from astrocytes or neurons in the developing POA is unknown. To further understanding of how PGE2 induces dendritic spine formation at the cellular level, we have explored the PGE2 receptor subtype mediating this response. There are four receptors for PGE2, EP1, EP2, EP3 and EP4, each having unique but interacting signal transduction profiles. Treatment of newborn female rats with the EP receptor agonists iloprost, butaprost and sulprostone indicated that stimulation of both the EP2 and EP3 receptors significantly increased spinophilin, a protein whose levels positively correlate to the presence of dendritic spines and masculinization of the POA. Use of antisense oligonucleotides against the mRNA for each receptor reveals that either EP2 or EP3 receptor knockdown reduces spinophilin in PGE2- or estradiol-treated females, whereas reducing EP1 or EP4 receptor levels by the same means has a smaller but also significant effect. A developmental profile of EP receptor expression indicates EP1 in particular is elevated for the first few days of life, corresponding to the critical period for masculinization, whereas mRNA levels for the other three receptors remain relatively constant.
Figures

References
-
- Adam M, Boie Y, Rushmore T, Muller G, Bastien L, Mckee K, Metters K, Abramovitz M. Cloning and expression of three isoforms of the human EP3 prostanoid receptor. FEBS Lett. 1994;338:170–174. - PubMed
-
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: Sex differences, regional heterogeneity and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. - PubMed
-
- Amateau SK, McCarthy MM. Sexual differentiation of astrocyte morphology in the developing rat preoptic area. J Neuroendocrinol. 2002a;14:904–910. - PubMed
-
- Amateau S, McCarthy M. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neuroscience. 2004;7:643–650. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

