Interphase chromosome arrangement in Arabidopsis thaliana is similar in differentiated and meristematic tissues and shows a transient mirror symmetry after nuclear division
- PMID: 17409060
- PMCID: PMC1894613
- DOI: 10.1534/genetics.107.073270
Interphase chromosome arrangement in Arabidopsis thaliana is similar in differentiated and meristematic tissues and shows a transient mirror symmetry after nuclear division
Abstract
Whole-mount fluorescence in situ hybridization (FISH) was applied to Arabidopsis thaliana seedlings to determine the three-dimensional (3D) interphase chromosome territory (CT) arrangement and heterochromatin location within the positional context of entire tissues or in particular cell types of morphologically well-preserved seedlings. The interphase chromosome arrangement was found to be similar between all inspected meristematic and differentiated root and shoot cells, indicating a lack of a gross reorganization during differentiation. The predominantly random CT arrangement (except for a more frequent association of the homologous chromosomes bearing a nucleolus organizer) and the peripheric location of centromeric heterochromatin were as previously observed for flow-sorted nuclei, but centromeres tend to fuse more often in nonendoreduplicating cells and NORs in differentiated cells. After mitosis, sister nuclei revealed a symmetric arrangement of homologous CTs waning with the progress of the cell cycle or in the course of differentiation. Thus, the interphase chromosome arrangement in A. thaliana nuclei seems to be constrained mainly by morphological features such as nuclear shape, presence or absence of a nucleolus organizer on chromosomes, nucleolar volume, and/or endopolyploidy level.
Figures
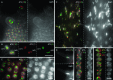
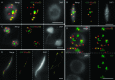

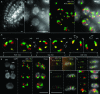
References
-
- Bártová, E., and S. Kozubek, 2006. Nuclear architecture in the light of gene expression and cell differentiation studies. Biol. Cell 98 323–336. - PubMed
-
- Bauwens, S., K. Katsanis, M. Van Montagu, P. Van Oostveldt and G. Engler, 1994. Procedure for whole mount fluorescence in situ hybridization of interphase nuclei on Arabidopsis thaliana. Plant J. 6 123–131.
-
- Belmont, A. S., 2006. Mitotic chromosome structure and condensation. Curr. Opin. Cell Biol. 18 632–638. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

