Control of sigma virus multiplication by the ref(2)P gene of Drosophila melanogaster: an in vivo study of the PB1 domain of Ref(2)P
- PMID: 17409092
- PMCID: PMC1893033
- DOI: 10.1534/genetics.106.063826
Control of sigma virus multiplication by the ref(2)P gene of Drosophila melanogaster: an in vivo study of the PB1 domain of Ref(2)P
Abstract
Ref(2)P has been described as one of the Drosophila proteins that interacts with the sigma virus cycle. We generated alleles to identify critical residues involved in the restrictive (inhibiting viral multiplication) or permissive (allowing viral multiplication) character of Ref(2)P. We demonstrate that permissive alleles increase the ability of the sigma virus to infect Drosophila when compared to null alleles and we confirm that restrictive alleles decrease this capacity. Moreover, we have created alleles unfunctional in viral cycling while functional for Ref(2)P fly functions. This type of allele had never been observed before and shows that fly- and virus-related activities of Ref(2)P are separable. The viral status of Ref(2)P variants is determined by the amino-terminal PB1 domain polymorphism. In addition, an isolated PB1 domain mimics virus-related functions even if it is similar to a loss of function toward fly-related activities. The evolutionary tree of the Ref(2)P PB1 domain that we could build on the basis of the natural allele sequences is in agreement with an evolution of PB1 domain due to successive transient selection waves.
Figures

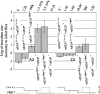
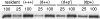
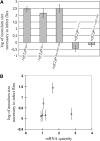

References
-
- Ayala, F. J., and C. A. Campbell, 1974. Frequency-dependent selection. Annu. Rev. Ecol. Syst. 5: 115–138.
-
- Bichon, A., N. Boukhatem, P. Gay, P. Dru, H. Terzian et al., 2001. Genetic and molecular features of Su(P), a gene that interacts with ref(2)P in male fertility of Drosophila melanogaster. Mol. Genet. Genomics 265: 354–361. - PubMed
-
- Brun, G., and N. Plus, 1980. The viruses of Drosophila, pp. 625–702 in The Genetics and Biology of Drosophila, edited by M. Ashburner and T. R. F. Wright. Academic Press, London.
-
- Bussereau, F., 1970. Study of CO2 sensitivity symptom induced by sigma virus in Drosophila. I. Influence of inoculation place on delay of symptom appearance. Ann. Inst. Pasteur (Paris) 118: 367–385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

