Herpes simplex virus latency-associated transcript sequence downstream of the promoter influences type-specific reactivation and viral neurotropism
- PMID: 17409161
- PMCID: PMC1900114
- DOI: 10.1128/JVI.02701-06
Herpes simplex virus latency-associated transcript sequence downstream of the promoter influences type-specific reactivation and viral neurotropism
Abstract
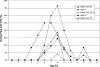
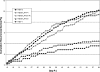
Herpes simplex virus (HSV) establishes latency in sensory nerve ganglia during acute infection and may later periodically reactivate to cause recurrent disease. HSV type 1 (HSV-1) reactivates more efficiently than HSV-2 from trigeminal ganglia while HSV-2 reactivates more efficiently than HSV-1 from lumbosacral dorsal root ganglia (DRG) to cause recurrent orofacial and genital herpes, respectively. In a previous study, a chimeric HSV-2 that expressed the latency-associated transcript (LAT) from HSV-1 reactivated similarly to wild-type HSV-1, suggesting that the LAT influences the type-specific reactivation phenotype of HSV-2. To further define the LAT region essential for type-specific reactivation, we constructed additional chimeric HSV-2 viruses by replacing the HSV-2 LAT promoter (HSV2-LAT-P1) or 2.5 kb of the HSV-2 LAT sequence (HSV2-LAT-S1) with the corresponding regions from HSV-1. HSV2-LAT-S1 was impaired for reactivation in the guinea pig genital model, while its rescuant and HSV2-LAT-P1 reactivated with a wild-type HSV-2 phenotype. Moreover, recurrences of HSV-2-LAT-S1 were frequently fatal, in contrast to the relatively mild recurrences of the other viruses. During recurrences, HSV2-LAT-S1 DNA increased more in the sacral cord compared to its rescuant or HSV-2. Thus, the LAT sequence region, not the LAT promoter region, provides essential elements for type-specific reactivation of HSV-2 and also plays a role in viral neurotropism. HSV-1 DNA, as quantified by real-time PCR, was more abundant in the lumbar spinal cord, while HSV-2 DNA was more abundant in the sacral spinal cord, which may provide insights into the mechanism for type-specific reactivation and different patterns of central nervous system infection of HSV-1 and HSV-2.
Figures




References
-
- Bustos, D. E., and S. S. Atherton. 2002. Detection of herpes simplex virus type 1 in human ciliary ganglia. Investig. Ophthalmol. Vis. Sci. 43:2244-2249. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

